Agile Medical Device Software Development
Drastically Reduce Time to Market & Adapt to Change Faster Than Your Competitors
Orthogonal applies Agile software development methods to medical device software development, allowing us to move faster while still delivering the quality, safety and reliability your device needs.
Achieve faster results by extending Agile
throughout the product lifecycle.
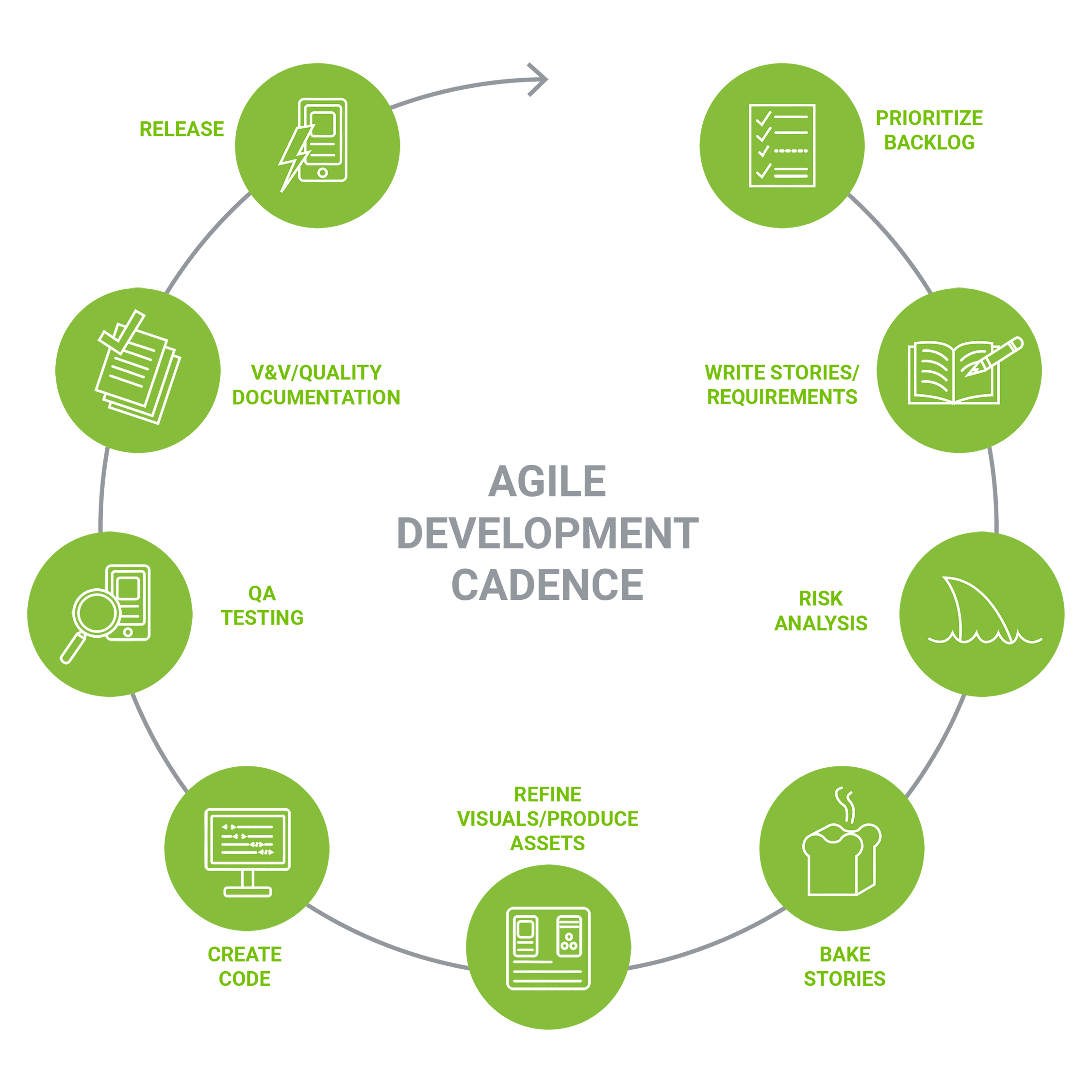
Software development projects are characterized by progressive learning. By adapting modern software development methods to medical device regulatory constraints, Orthogonal’s teams can flexibly address new information and insights while maintaining project velocity and ensuring compliance with global standards.
Agile practices inform not only software development, but also our approach to integrating UX, risk management, cybersecurity and verification & validation. We successfully deal with uncertainty and mitigate risk using fast feedback loops. Working in iterative cycles prior to and in parallel with development, we get feedback early and often, letting our clients create better products faster.
Evolve
Agile practices beyond launch
power more frequent releases.
The strengths of Agile allow you to continuously evolve your product after it hits the market. By developing in small increments, you can incorporate market feedback more quickly, leading to faster improvement of your medical device software.

Our QMS
Quality, agility and compliance.
We do Agile development within our ISO 13485:2016 Quality Management System, and our practices are compliant with IEC 62304, ISO 14971 and AAMI TIR:45. All together, you get the benefits of Agile and the confidence of being compliant with regulatory standards.

Agile methods are enabled or complemented by:
Agile eBook
We wrote the book on Agile
for medical device software. Literally.
Orthogonal is a thought leader in applying Agile development methods to the creation of highly regulated Software as a Medical Device, DTx and connected medical device systems. This groundbreaking approach is covered in our “Agile in an FDA Regulated Environment” eBook.
