“Applying modern engineering techniques to the development of SaMD is no easy feat. Orthogonal’s approach to blending these domains helped Tandem accelerate our product innovation cycle and outputs”
Case Study
Orthogonal’s medical device software development services encompass the full product lifecycle, including user experience design, human factors, requirements definition, risk analysis, Agile software development, and verification and validation. We’ll help you build and evolve game-changing medical device software, SaMD and connected devices faster than the competition.
“Applying modern engineering techniques to the development of SaMD is no easy feat. Orthogonal’s approach to blending these domains helped Tandem accelerate our product innovation cycle and outputs”
“From day one, Orthogonal helped us methodically achieve regulatory quality. Today, our QMS is core to our DNA. It’s a strategic asset in the highly complex and competitive DTx space.”
With over a decade of experience, Orthogonal is uniquely qualified to help you take advantage of the rapidly expanding opportunities presented by digital technologies. We enable you to not just build the right medical device software faster, but to evolve faster and scale faster than your competitors.
Unlike other companies in our field, which have added “software” to their repertoires over time, software has always been at Orthogonal’s core – and our track record of work and thought leadership is a testament to that.
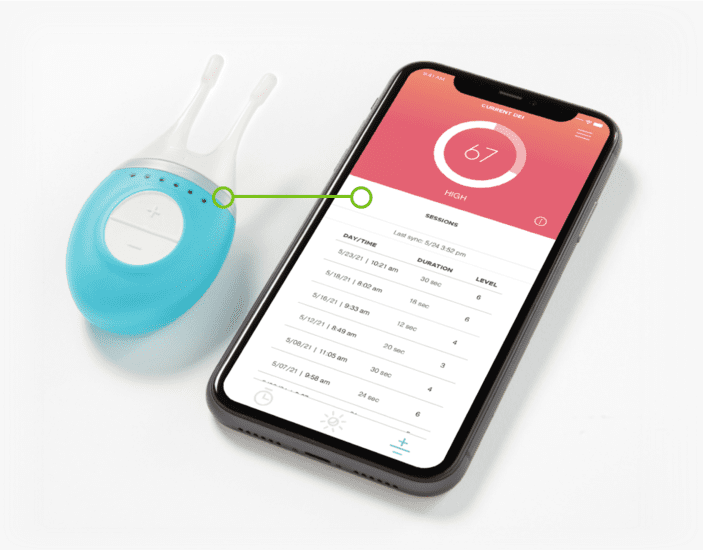
We’ve developed medical device software for Class II and Class III connected medical devices, with functionality including remote patient monitoring, remote patient care delivery via smart therapeutics (insulin pumps, nerve stimulators) and point of care in vitro diagnostics (IVDRs).
Orthogonal has deep experience developing SaMD products that take the form of mobile apps, web apps, desktop apps cloud computing, AI algorithms and SDKs for integration to 3rd party platforms. Our past projects include Digital Therapeutics (DTx), digital diagnostics and digital biomarkers.
Our Agile processes are designed for efficiency so you can deliver software faster and with higher quality.
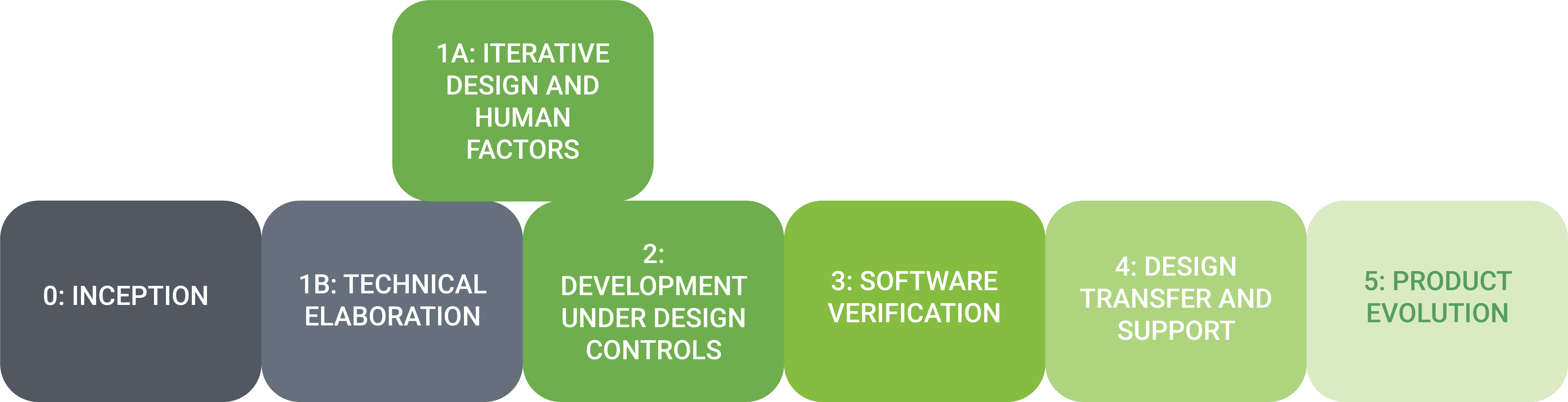
Phase 0: Inception: A series of workshops that lay the technical, design and quality foundations of the medical device software and get the team working together.
Phase 1a: Iterative Design and Human Factors: Brings the medical device software design from storyboard to high fidelity screens, with highly integrated user testing to add in the user’s voice, needs and preferences early in the process, as well as meet human factors compliance requirements.
Phase 1b: Technical Elaboration: Firms up key architectural aspects of the medical device software system and addresses key domains such as cybersecurity, risk analysis, quality and regulatory.
Phase 2: Development Under Design Controls: The Agile process for bringing medical device software to life. Sprints are typically two weeks and follow a cadence of baking, grooming, planning and execution.
Phase 3: Software Verification: Performs formal verification activities as outlined in the Software Verification Strategy. These activities are performed iteratively in the specification and development phases, resulting in a faster final verification for the medical device software.
Phase 4: Design Transfer and Support for System Testing: Provides support for the final User Acceptance Test (UAT) and transfer of medical device software source code, build files, quality documentation and any needed knowledge transfer.
Phase 5: Evolution: Incorporates real world feedback into rapid release cycles that propels medical device software further and faster than competitors.
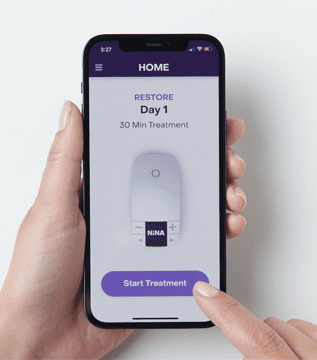
Case Study
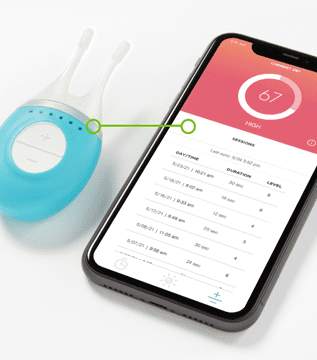
Case Study
Case Study

Case Study
We’ve spent a decade refining our approach to building complex SaMD and medical device software, so we can focus on meeting the unique technical challenges of your solution.
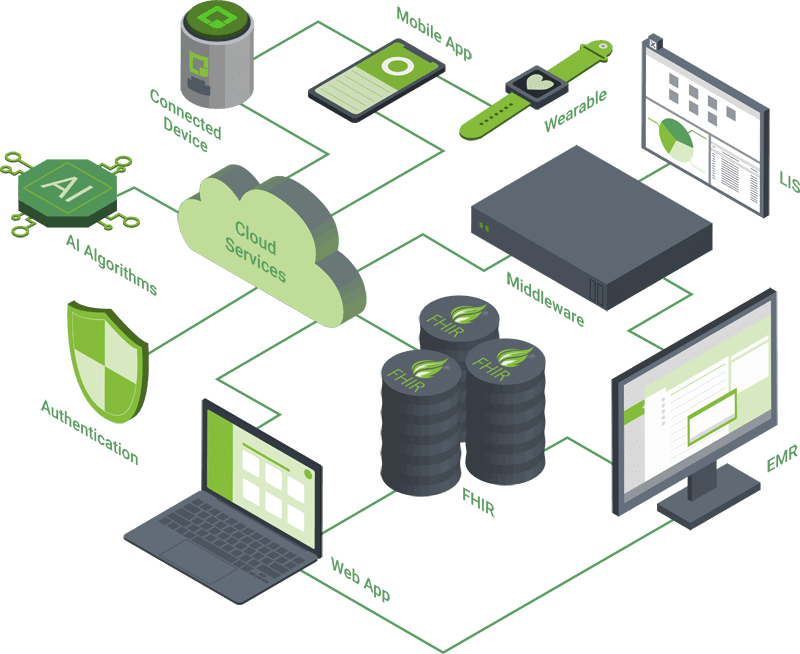
Orthogonal has developed dozens of medical device mobile applications for both iOS and Android, from standalone SaMD to continuous glucose monitors to device control apps for Class III active implantables. Working with both native development and cross-platform development tools, such as Flutter and React Native, we ensure support for a wide range of mobile devices in an ever-shifting BYOD environment.

Orthogonal builds modern web applications using React and Flutter – from simple dashboards, to multi-layered apps with rendering layers, domain logic and multiple types of users. We apply clean architecture principles to ensure separation of concerns, resulting in client-side applications that are robust, testable, flexible and maintainable.

Orthogonal specializes in developing mobile applications and cloud services that connect to implanted and wearable devices using Bluetooth, cellular and NFC technologies. With our extensive library of corner cases, testing methods and mitigations, we get the most out of wireless communication, maximizing reliability and speed while minimizing power consumption.
Orthogonal has extensive experience applying techniques like auto-scaling by tier, self-healing and health and performance checks for Amazon Web Services (AWS), Microsoft Azure and Google Cloud Platform (GCP). We balance performance, scalability and data security with regulatory and standards compliance. We collaborate with the FDA, major cloud providers and other medical device companies in shaping cloud regulations and guidance; we are co-chairs of the AAMI working group developing Technical Information Report (TIR) #115.

Orthogonal has developed a number of FDA-cleared AI and machine learning algorithms – and we’re involved in the ongoing development of AI-related standards. We use technologies and tools such as Tensor Flow and Tensor Flow Lite, data case management, signal processing analysis, OpenCV and digital signal processing. We undertake algorithm development to create algorithms for feature identification, multidimensional signal processing, cardiac signal analysis and general-purpose signal processing.

Integration of SaMD with EMR systems is a critical part of the care delivery process. Orthogonal leverages middleware solutions that provide single uniform endpoints to different EMR instances to streamline integration. We’ve built out high-performance cloud infrastructure for mobile and web apps using FHIR, DICOM and SMART on FHIR for seamless and secure interoperability. For FHIR data storage, we tailor our approach to the platform: Cloud Healthcare API and FHIR stores for GCP; FHIR Service on Azure Health Data Services; and FHIR Works on AWS.

Cybersecurity is an ongoing activity that is woven into Orthogonal’s product development lifecycle. Our process is compliant with the FDA’s pre- and post-market cybersecurity guidance for medical devices, as well as AAMI TIR57:2016, ISO/IEC 27001 and the NIST framework, and continues to evolve along with the SaMD ecosystem and regulatory landscape.

“Orthogonal brought us the expertise we needed to ensure our technology platform met the high standards of the FDA. Through their involvement, we absolutely accelerated our pace through the regulatory process. ”
“We needed a SaMD partner equally versed in medical device software development and modern software engineering. Orthogonal clearly crosses that bar, and has been an invaluable asset for Boehringer Ingelheim Digital.”
“Applying modern engineering techniques to the development of SaMD is no easy feat. Orthogonal’s approach to blending these domains helped Tandem accelerate our product innovation cycle and outputs”