Case Study
Neurostimulation Device for Dry Eye Disease
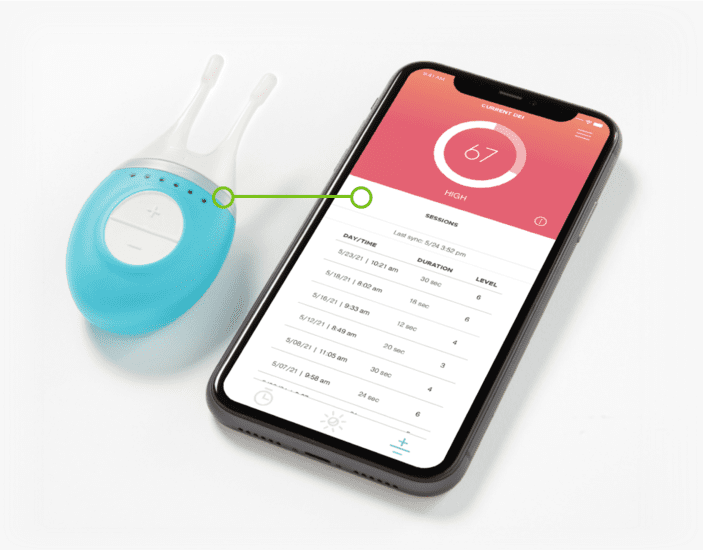
Device and app connectivity
Executive Summary
Orthogonal partnered with our client, a large pharmaceutical firm who had recently purchased a venture-backed startup, to develop a complete connected medical device solution for their Class II therapeutic nerve stimulation device. The device naturally stimulated the production of tears in the eye to treat chronic dry eye disease.
We designed and built iOS and Android companion apps for our client’s device that patients could download onto their own Apple and Android smartphones. The mobile app connected over Bluetooth Low Energy to the nerve stimulation device, and used device data, smartphone location data as well as weather and pollution data pulled from cloud back end services as inputs for an algorithm that provided personalized dry eye forecasts to the end user.
The companion mobile app was designed to organically drive strong user adoption and ongoing engagement by leveraging patient-generated data, as well as data collected from the device. Our app not only used patient data to help feed the previously mentioned algorithm, but also gave that data back to patients in a context that helped them better understand and manage their condition.
As with many suffering from chronic conditions, patients placed a high value on data that provided them with a sense of control over their dry eye disease. Because of this feedback loop, patients were more likely to use the device regularly, resulting in better outcomes for them and better data for our client.
The Medical Issue
Dry eye disease¹ affects 16 million people in the U.S. alone. It is a chronic condition wherein the eyes either fail to produce enough tears to keep themselves damp, or the tears that are produced don’t have the right mix of biological ingredients that the eyes need. It can cause blurred vision, eye redness and irritation, stinging and burning sensations – unpleasant symptoms that have a large impact on quality of life. Furthermore, symptoms can be worsened by environmental triggers such as weather and air quality.
Despite the prevalence of dry eye disease, there is no single solution with a high success rate that is also affordable and easy to use. The primary treatment for the disease is artificial tears (AT), which come in over-the-counter and stronger prescription varieties. While AT can help soothe mild symptoms, a survey of doctors found that it worked well for 60-80% of patients, meaning anywhere from 20% up to 40% of patients received no benefit from AT2.
Other treatments for dry eye disease include autologous serum, which is distilled from a patient’s own blood; punctual occlusion, which places a plug in the patient’s eye; and rigid gas permeable scleral lenses, which are hard, thick contact lenses that are recommended for patients with severe symptoms3. On the whole, the available treatments for dry eye disease can be hard to use, expensive and can interrupt a patient’s daily life.
The Opportunity
Our client was a large pharmaceutical firm who recently purchased a startup. The acquired startup had seen an opportunity for a breakthrough approach that bypassed pharmaceutical eye drops of synthetic tears: a small, portable medical device that patients could use to gently stimulate the nerves that naturally generate tears.
This form of treatment is known as neurostimulation – a promising new approach to delivering non-invasive but highly effective therapies to the body. The global neurostimulation device market was valued at $5.21 billion in 2020; analysts project it to grow to $11.28 billion by 20264.
Neurostimulation has the potential to be effective in a variety of applications. For example, during the coronavirus pandemic, a non-invasive vagus nerve5 stimulation device was granted emergency use authorization by the FDA to treat patients with COVID-19 who displayed exacerbated athsmatic symptoms6. Further research into neurostimulation could lead to innovations in treating epilepsy, major depressive disorder, cluster headaches and post-stroke complications7.
Our client’s research demonstrated that a nerve stimulation treatment for dry eye disease had far greater success rates with patients and shed many of the challenges of carrying around prescription eye drops. In addition, it could increase patient comfort, be applied more prophylactically than eye drops and generally have a positive impact on a patient’s overall quality of life.
Investing in non-invasive medical device solutions was a major step for our client, as they transformed from a pharmaceutical company, one that developed and sold medication for treating diseases, into a therapeutics company that provided the best solutions to important health issues. A connected medical device, including a companion app and cloud back end, allowed the firm to maintain an ongoing relationship with the patient and their doctor – a relationship that could improve the quality of life for tens of millions of patients.
This transformation is a great example of how the boundary lines between a traditional pharmaceutical firm and a medical device company are being blurred, in part by the digitization of healthcare. Embracing software-enabled therapies allows pharmaceutical firms to stay in the forefront of the solution space, while those that hew to an older approach risk losing market share. Staying relevant in healthcare means becoming a software company. To quote Marc Andreessen, “Software is eating the world” – a statement which we wholeheartedly agree with and discuss in this article8.
The Challenge and Solution

Nerve stimulation device’s components
The core challenge for our client was that they didn’t have a medical device-focused quality management system, let alone one that could accommodate a Bluetooth-enabled device with a companion mobile app. They needed Orthogonal’s help to create an app that would guide the user in proper use of the device, encourage its frequent use and demonstrate the effectiveness of the therapy.
We worked with our client to develop a complete connected medical device solution, focusing on a new smartphone application that connected via Bluetooth Low Energy (BLE) to the nerve stimulation device on one side, and over the Internet to the cloud on the other side.
The app supported a wide range of features, including initial user registration of the device with the client’s online patient services, an algorithm that provided a daily Dry Eye Index as well as a Dry Eye 5-Day Forecast to help patients predict on which upcoming days flare-ups were most likely. This algorithm was fed a variety of disparate information, including patient profile data, the patient’s location and third-party data such as area weather and air pollution forecasts.
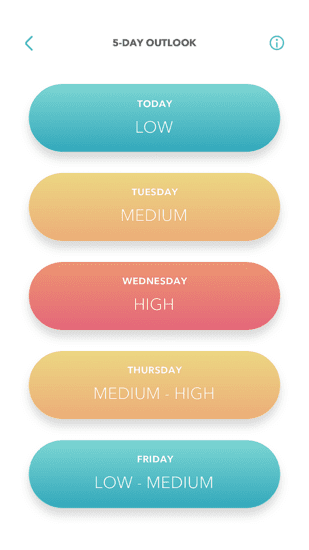
5-day Dry Eye Forecast
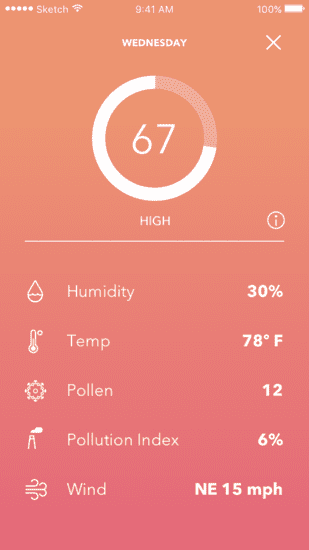
Dry Eye Index screen shows factors that went into forecast
As a connected medical device system, the overall solution can create data as a byproduct of its use. This data could be aggregated by the manufacturer to demonstrate clinical effectiveness – both to incentivise patients to stick with the treatment and to justify reimbursement by payers.
Patient Engagement and Habit Formation
Orthogonal’s approach to designing companion software for connected medical devices (as well as Software as a Medical Device (SaMD) and Digital Therapeutics (DTx)) is rooted in patient empathy. Creating a medical device that works is only the beginning. Determining key experiential priorities early on is critical to hooking patients into using it regularly. In collaboration with the project stakeholders, we decided upon the following priorities: the patient’s setup and initial use experience; ensuring correct use of the device; and measuring the long-term evolution of the patient’s condition.
These experiential priorities became the basis of scope for our user research. Our goal was to discover and verify the features that would most positively contribute to the project’s priorities through iterative in-person user testing. The reason for identifying these features was to make our app sticky: able to provide value to the patient while getting data back from them in return. That data would certainly be useful to the manufacturer, but combined with the right information, it could also be useful to the patient – allowing the app to organically drive strong user adoption and ongoing engagement.
Two key insights stood out from the user experience design efforts. First, we were initially concerned about patient’s willingness to provide feedback via an in-app validated instrument set up to collect Patient Recorded Outcomes. We discovered that they were very willing to share information about their condition if the information was provided back to the patients as part of progress reports. As with many suffering from chronic conditions, they placed a high value on data that provided them with a sense of control over their condition.

App screens simulated on smartphone
Second, as mentioned earlier, dry eye disease has environmental triggers. We addressed this by combining data relating to smartphone location, device usage, patient feedback, local weather and pollution. Doing so allowed us to provide patients with their personalized Dry Eye Index and 5-Day Forecast. This feature tested very well with users during the design process, and product analytics and qualitative post-market feedback showed it to be one of the most popular components of the application. When users feel an immediate sense of satisfaction from managing their condition, it drives the adoption of the therapy and makes the treatment habit-forming.
Technical Solutions
Our teams set out to develop the following:
- iOS and Android mobile apps that synced with the BLE-enabled neurostimulation device
- Back end infrastructure, analytics, algorithms and administrative interfaces
- Integration with pollution and weather data feeds on Amazon Web Services (AWS)
A key technical challenge was how to optimize the synching of the device with the phone over BLE in a way that was user-friendly and highly reliable in a variety of settings and scenarios.
An aggressive timeline for product launch drove parallel development of all components of the solution, including the physical nerve stimulation device and the electronics inside, device firmware, connectivity protocols, the mobile apps and the cloud infrastructure.
Device website mobile app section
To mitigate risk of these parallel but interdependent activities happening at a fast pace, Orthogonal built out an integration testing infrastructure that allowed each component to be continually tested, first against simulations of the other components, and later against the actual components as they were available.
By adopting this Agile approach early, and continuously testing and retesting all aspects of the product, our teams were able to quickly identify and resolve integration issues that otherwise could have added an entire year to the development schedule.
Technical Services
System Testing Harness Development: Orthogonal developed an Android-based testing application and AWS back end to test functionality via BLE connectivity for the original prototype device developed by the client. We maintained and extended the testing harness as device functionality, communication protocols and mobile and cloud functionality evolved. Some of the features of the testing harness included:
- BLE protocol library for iOS and Android
- Device fuzz testing
- Stress testing the device with edge cases
- Regression testing for increment data
- BLE pairing and disconnection
Manufacturing Control Android App: Extension of the testing harness allowed manufacturing users to enter serial number, program device data and erase patient data on the selected devices, and to move the device into commercial mode, all via BLE.
Protocol Design and Development: The Bluetooth protocol developed for the prototype device was designed for extracting all data on the device in one shot. Commercial use would require more frequent communication of smaller data sets, as well as encryption. Therefore, developing, testing and refining BLE protocols for the device was of high importance.
Bluetooth Firmware Development: We implemented the BLE protocols we designed in firmware and performed a number of firmware optimizations.
Algorithm Development: Orthogonal worked with the client to develop the Dry Eye Index and Personalized Dry Eye Forecast algorithms.
iOS and Android Patient Applications: Orthogonal developed patient-facing iOS and Android applications to interface with the device via BLE, including development of the BLE libraries.
Amazon Web Services Back End: Orthogonal developed back end functionality on AWS, including administrative and analytic web interfaces for our client’s researchers.
Risk Management: Orthogonal participated in the client’s risk management process by providing software and use error risk analysis, as well as proposed risk mitigations. Orthogonal relied on the client for clinical risk evaluation.
Cybersecurity Risk Management: Orthogonal performed a number of cyber security risk management activities in accordance with AAMI TIR57: 2016, Principles for medical device security—Risk management9 and FDA pre10 and post market11 cybersecurity guidance. Some of the activities we performed included threat modeling and mitigations, fuzz testing, penetration testing and Amazon well-architected reviews.
Learn More
To learn more about the services and technologies Orthogonal performed in this case study, please visit:
- Our Services
- Our Technologies
- Agile in an FDA Regulated Environment white paper
- Software as a Medical Device (SaMD): What It Is & Why It Matters white paper
- Patient Engagement and SaMD – Orthogonal speaks at Med Device Summit
- Patient Engagement and Medical-Grade Lifestyle Devices
References 1. Dry Eye | National Eye Institute. Nei.nih.gov. https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/dry-eye. Published 2020. Accessed November 30, 2021. 2. Williamson J, Huynh K, Weaver M, Davis R. Perceptions of Dry Eye Disease Management in Current Clinical Practice. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4440861/ Published 2014. Accessed November 30, 2021. 3. Ding PhD J. patients - When Artificial Tears just don’t cut it – other Treatments of Dry Eye - TFOS - Tear Film & Ocular Surface Society. Tearfilm.org. 4. Neurostimulation Devices Market Report Share & Size. Delveinsight.com. https://www.delveinsight.com/report-store/neurostimulation-devices-market?utm_source=globenewswire&utm_medium=pressrelease&utm_campaign=ppr. Published 2021. Accessed December 1, 2021. 5. Seladi-Schulman PhD J. Vagus Nerve: Function, Stimulation, and More. Healthline. https://www.healthline.com/human-body-maps/vagus-nerve. Published 2021. Accessed December 1, 2021. 6. LLP D. Neurostimulation Devices Market is Anticipated to Grow at a CAGR of 11.76% and will touch USD 11.28 billion by 2026, States DelveInsight. GlobeNewswire News Room. https://www.globenewswire.com/en/news-release/2021/09/30/2305842/0/en/Neurostimulation-Devices-Market-is-Anticipated-to-Grow-at-a-CAGR-of-11-76-and-will-touch-USD-11-28-billion-by-2026-States-DelveInsight.html. Published 2021. Accessed December 1, 2021. 7. Howley E. What Is Vagus Nerve Stimulation?. https://health.usnews.com/health-care/patient-advice/articles/what-is-vagus-nerve-stimulation. Published 2021. Accessed December 1, 2021. 8. Kappe B. 10 Year Anniversary of 'Software is Eating the World' | Orthogonal.io. Orthogonal. https://orthogonal.io/insights/a-decade-of-software-eating-the-world-of-medical-devices/. Published 2021. Accessed December 1, 2021. 9. AAMI TIR57: 2016 - Principles for medical device security - Risk management. Webstore.ansi.org. https://webstore.ansi.org/standards/aami/aamitir572016. Accessed December 1, 2021. 10. Premarket Submissions: Management of Cybersecurity in Medical Devices. U.S. Food and Drug Administration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/content-premarket-submissions-management-cybersecurity-medical-devices. Published 2018. Accessed December 1, 2021. 11. Postmarket Management of Cybersecurity in Medical Devices - Guidance. U.S. Food and Drug Administration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/postmarket-management-cybersecurity-medical-devices. Published 2016. Accessed December 1, 2021