Webinar
Medical devices connected to a smartphone through Bluetooth Low Energy (BLE) create new possibilities for patient monitoring and device performance. BLE offloads functionality from the device to the app, lowering physical hardware costs and creating software solutions that are powerful, sophisticated and easy to use. BLE-enabled systems can control pumps and implants, send real-time alerts to patients and pull data from the cloud with 1/10th of the power consumption of WiFi and significantly lower consumption than Bluetooth Classic.

Wireless-enabled medical devices and companion apps run in the background of a smartphone, allowing patients to focus on their lives, not their devices. But because background processing is controlled by the mobile operating system, it takes specialized knowledge to maximize reliability while minimizing risks. Orthogonal has developed an extensive library of edge cases over our decade of experience optimizing BLE connectivity, letting us design for, test for and gracefully recover from any issue.
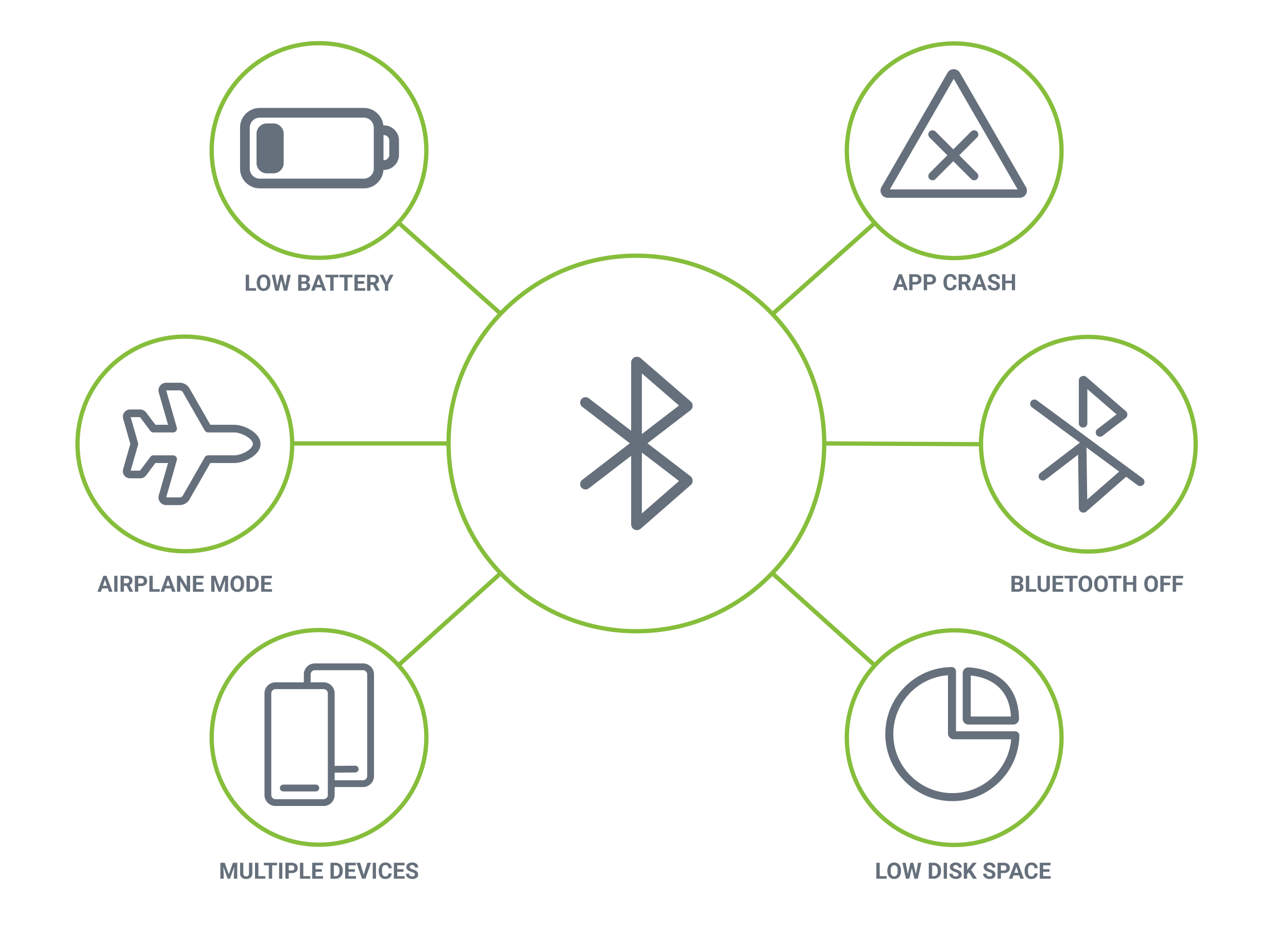
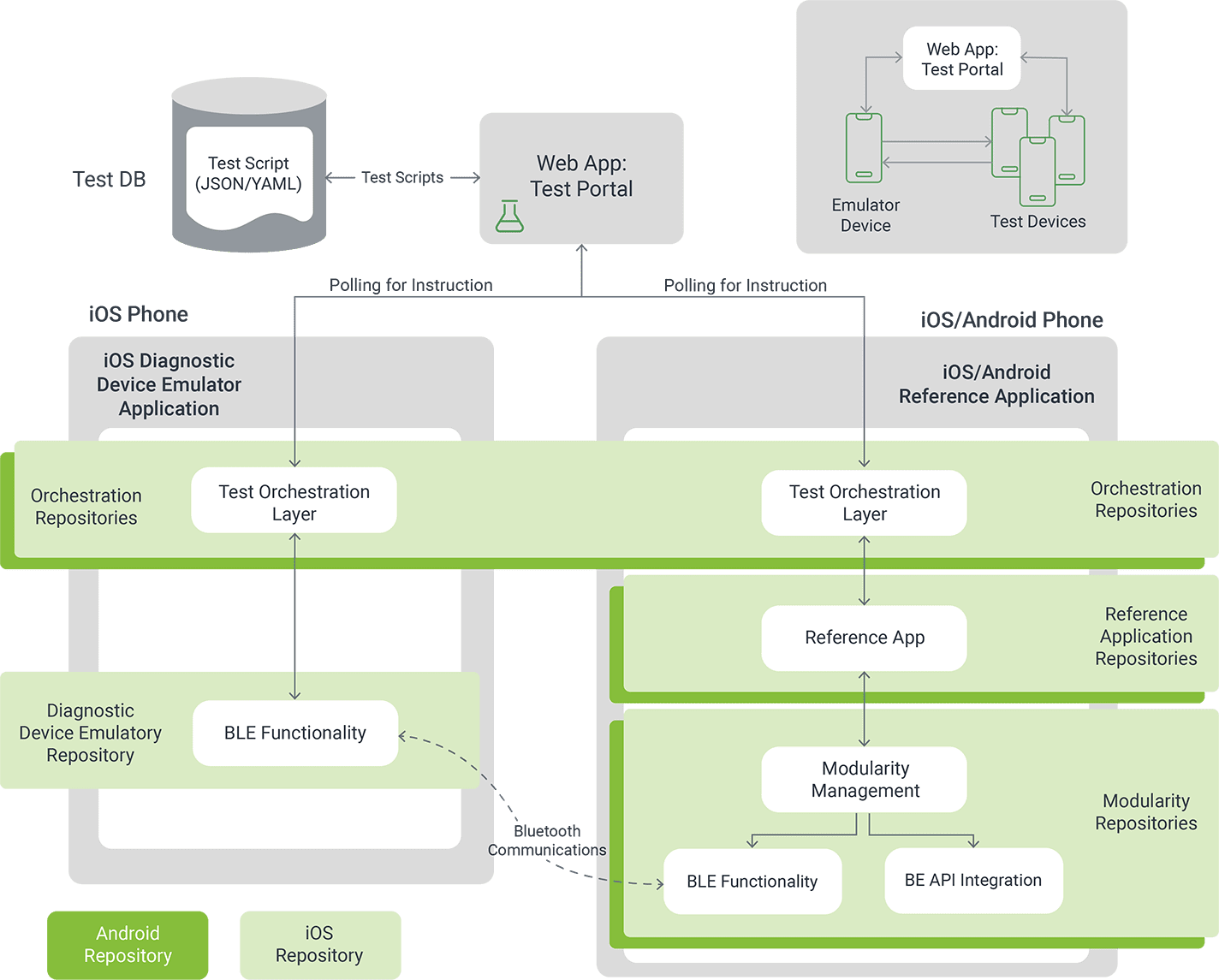
Orthogonal takes a layered approach to edge case testing, sized to the scope of your device. We take into account the complexity of the BLE connection, the quantity and frequency of data sent between the app and device, and the number of smart device profiles your app will work on. That informs which edge cases we’ll test against your software, using tools like cloud testing farms, device simulators and actual smartphone hardware. We’ll work with you to navigate the complex Bring Your Own Device market and mitigate risks your connected device may encounter.
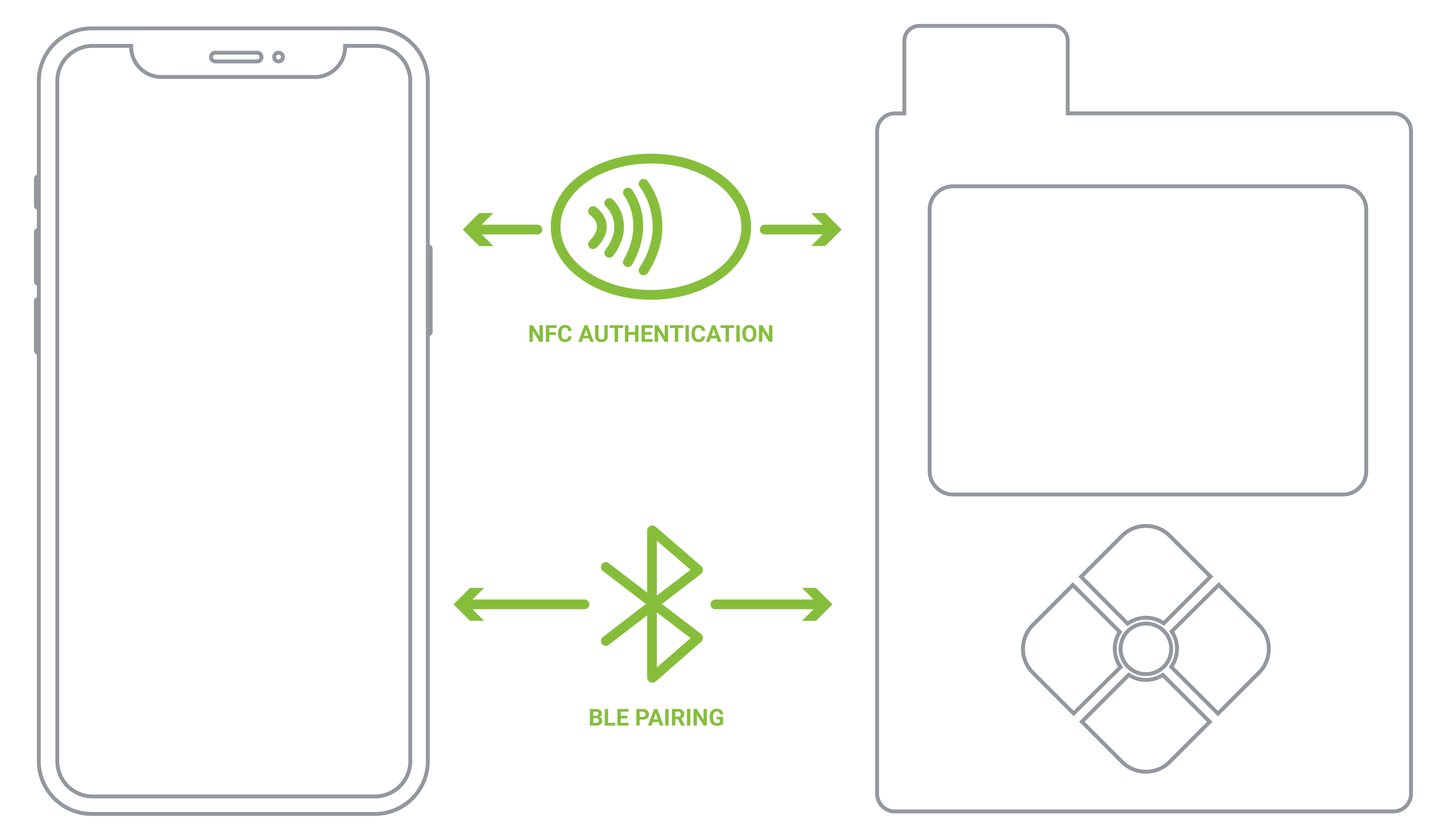
Connectivity between a smartphone and a medical device, and by extension a smartphone and the cloud, adds increased cybersecurity risk. Orthogonal employs cybersecurity risk planning and risk management both during development and after release to reduce attack surfaces. We apply best practices to limit pairing windows, such as Out-Of-Band pairing using NFC, to minimize risks during the pairing process, and forcing encryption of all data over the air and at the app level where necessary.
BLE enables next-gen companion applications with powerful functionalities, but implementing it is a challenge due to smartphone complexities. Orthogonal has been solving the hard problems around device and smartphone connectivity through BLE since its introduction. We’ll help you maximize performance and reliability while resolving technical and regulatory concerns.

White Paper
Smartphones are everywhere. Success for medical device developers and manufacturers lies in their ability to integrate their proprietary hardware devices with companion apps on users’ smartphones through Bluetooth. This connectivity paves the way for the creation of powerful, convenient and engaging digital health solutions. But to reap the clear benefits afforded by Bluetooth, one must first overcome significant challenges.
This white paper collects insights and best practices discussed in our 2023 Bluetooth webinar series, aiming provide a comprehensive strategy for medical device manufacturers and developers creating Bluetooth-enabled medical devices and companion apps.


Webinar
Webinar

Webinar
Webinar

Webinar

Webinar
Article

White Paper
Webinar

Article

White Paper

Article

Article