Talk
Future of Wireless Technology for Medical Devices: Webinar
MedTech makes use of advanced Bluetooth Low Energy (BLE) features in our Bluetooth-enabled medical devices, ones that require specialized technical knowledge to get right. You need experts on both the hardware and software side, so that when it’s time to link up the device hardware and the companion mobile app, they work together optimally. That’s why Orthogonal’s medical device software developers teamed up with the medical device hardware engineers at Blur on a recent project.
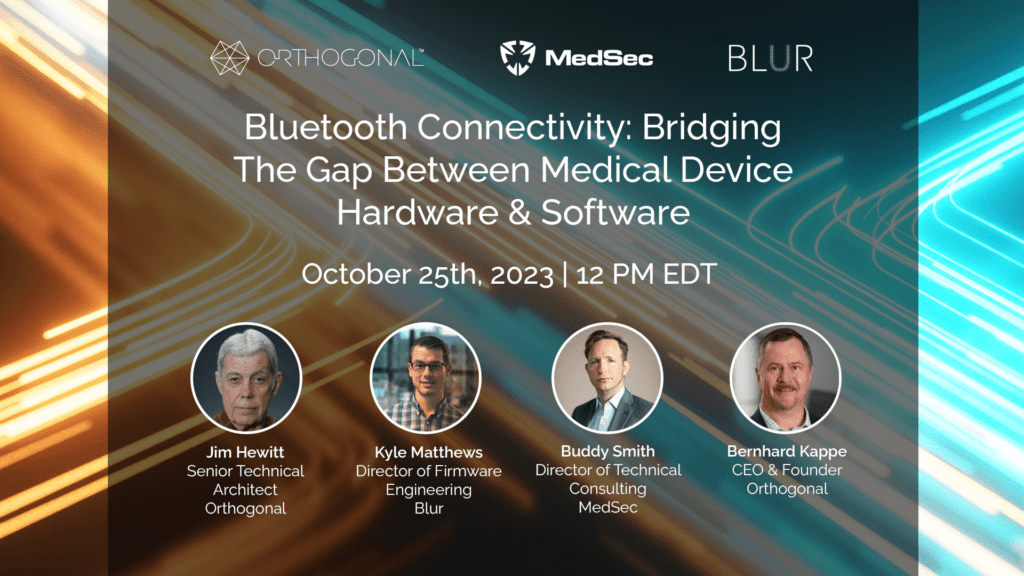
On October 25th, 2023, Orthogonal, Blur and MedSec held a webinar discussion on the collaboration between Orthogonal and Blur, part of our BLE for Medical Devices Webinar Series. Jim Hewitt, Senior Technical Architect at Orthogonal, and Kyle Matthews, Director of Firmware Engineering at Blur, shared their experiences leading the software and hardware teams respectively, and how they worked together to integrate the two sides of the BLE-enabled medical device. They were joined by Buddy Smith, Director of Technical Consulting at MedSec, Bernhard Kappe, Orthogonal’s Founder and CEO. The webinar was moderated by Randy Horton, Chief Solutions Officer at Orthogonal.
1. Collaboration between medical device hardware engineers and medical device software developers, both of whom have different experiences and optimization goals, requires compromise and flexibility. Teams have a tendency to drive simplification on their side and push complexity to the other side, as well as treat initial plans as being set in stone. To avoid these common pitfalls, it’s recommended to bring teams together as early in the development process as possible to align on what’s best for the system.
2. Another key practice is to establish open channels of communication. Our speakers’ teams created a Confluence page and a Slack channel to make collaboration instant and frictionless. They had daily discussions as well as weekly meetings that helped foster a collaborative environment. Openness to learning from each other allowed the teams to work together more smoothly.
3. Along with bringing the teams together at the start of development, it’s essential to establish a cybersecurity threat model upfront and do penetration tests early and often.
4. In the experience of the speakers, one of the biggest challenges in developing a Bluetooth-enabled medical device is balancing battery optimization on the hardware side and user experience on the software side. Hardware and firmware need to be optimized for battery life, but choices made to limit power consumption may slow down the communication between the device and smartphone. Depending on how the device uses Bluetooth, teams will need to come to a compromise between efficiency and usability.
5. The pace of development for firmware is slower than that for smartphone software, posing a challenge to coordinating Bluetooth activities. In our speakers’ projects, the smartphone software team created Bluetooth emulation applications that ran on a smartphone or computer so they could develop the app before the hardware and firmware became available to work on. Both teams had access to each other’s code repositories, enabling them to update software as commits were made.
6. Our speakers recommended building on previous experience when starting a new project. For example, it’s helpful to have knowledge and experience with the chosen chip and its ecosystem, as each has its own quirks.
Jim Hewitt, Senior Technical Architect, Orthogonal
Jim Hewitt provides deep expertise in software, firmware, data architecture, and development in health informatics and medical device projects for Orthogonal. He brings over 35 years of experience as a senior technical architect focusing on team and project success in a variety of industries, and he has worked on technically advanced – and sometimes ground-breaking – solutions with a variety of companies during his career. In addition, he has established himself as a leading expert on the use of Bluetooth technologies to connect medical devices to smartphone apps and the cloud.
Kyle Matthews, Director of Firmware Engineering, Blur Product Development
Kyle Matthews is a hardware and firmware engineer at Blur. There, he is responsible for complete hardware design, including architectural and systems design, schematic layout, PCB routing, board bring-up, firmware development and debugging for a variety of client projects. Kyle has researched and developed novel technologies, resulting in multiple patents for clients.
Buddy Smith, Director of Technical Consulting, MedSec
Buddy Smith is a security engineer focused on protecting electronic devices from attack. He has an extensive background in firmware development, bringing his passion for embedded development to the security world. In his 15 years of experience, Buddy has worked in cryptography, hardware design, firmware engineering, and information security. In his role at MedSec, he has supported clients with regulatory filings, performed penetration tests of devices and created threat models for systems, from long-lived implantable devices to bedside infusion pumps.
Buddy holds a Bachelor of Science in Computer Engineering from the Georgia Institute of Technology, and is an Offensive Security Certified Professional. He is also an IEEE Senior member.
Bernhard Kappe, CEO and Founder, Orthogonal
Bernhard Kappe is the Founder and CEO of Orthogonal. For over a decade, Bernhard has provided thought leadership and innovation in the fields of Software as a Medical Device (SaMD), Digital Therapeutics (DTx) and connected medical device systems. As a leader in the MedTech industry, Bernhard has a passion for launching successful medical device software that makes a difference for providers and patients, as well as helping companies deliver more from their innovation pipelines. He’s the author of the eBook Agile in an FDA Regulated Environment and a co-author of the AAMI Consensus Report on cloud computing for medical devices. Bernhard was the founder of the Chicago Product Management Association (ChiPMA) and the Chicago Lean Startup Challenge. He earned a Bachelor’s and Masters in Mathematics from the University of Pennsylvania, and a Bachelor’s of Science and Economics from the Wharton School of Business.
Randy Horton, Chief Solutions Officer, Orthogonal
Randy Horton is Chief Solutions Officer at Orthogonal, a software consulting firm that improves patient outcomes faster by helping MedTech firms accelerate their development pipelines for Software as a Medical Device (SaMD), digital therapeutics (DTx) and connected medical device systems. Orthogonal makes that acceleration happen by fusing modern software engineering and product management tools and techniques (e.g., Agile, Lean Startup, User-Centered Design and Systems Thinking) with the regulated focus on device safety and effectiveness that is at the heart of MedTech.
Horton serves as Co-Chair for AAMI’s Cloud Computing Working Group, as well as AAMI CR:510(2021) and the in-process Technical Information Report #115, all of which address how to safely move medical device computing functions into the cloud. He is a frequent speaker at conferences and webinars, including events hosted by AdvaMed, AAMI, HLTH, RAPS and the Human Factors and Ergonomics Society (HFES).
Related Posts

Talk
Future of Wireless Technology for Medical Devices: Webinar

Talk
Meeting FDA Requirements for BLE Mobile Medical Apps: Webinar
Talk
Solving Edge Cases for Bluetooth Medical Devices: Webinar Summary

Talk
Cybersecurity for Bluetooth Medical Devices: Webinar Summary