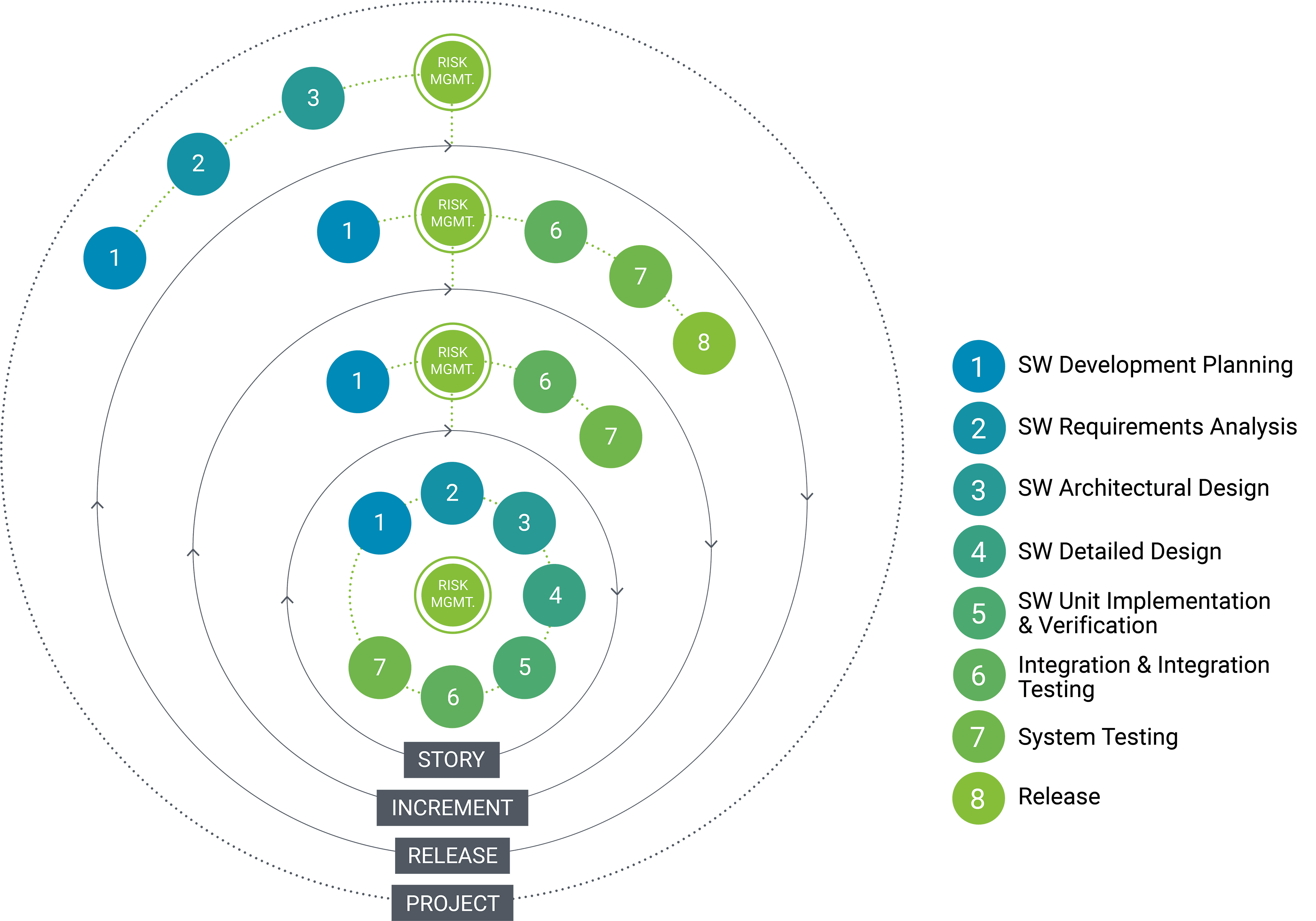
Agile + Risk Management
Iterative risk management powered by Agile.
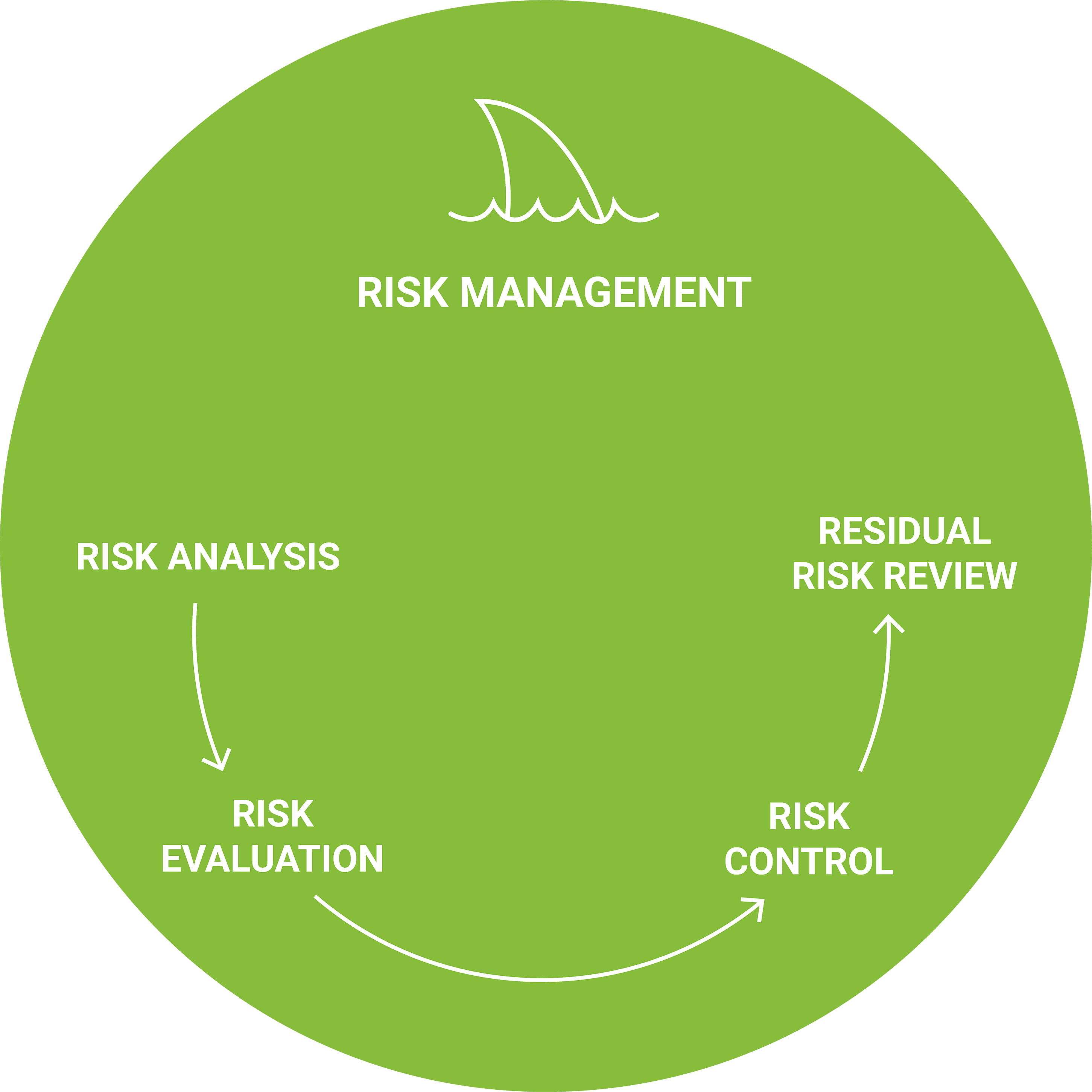
Risk management is baked into our ISO 14971 and IEC 62304-compliant Agile approach. Risk analysis, evaluation and control are performed in each sprint or iteration, speeding the discovery and mitigation of risk while maintaining project velocity and cost effectiveness. It’s how we ensure clarity, regulatory compliance and – most important of all – patient safety.
Human Factors + Risk Management
Fast feedback to minimize use errors.
Many of the risks for connected medical devices are directly related to human factors (HF), including permitted misuses, permitted user complacency and user interface designs that are not adapted to user workflows. Orthogonal integrates HF engineering into risk management. With fast feedback loops and frequent iterative testing of wireframes, task flows and storyboards, we can identify and mitigate risks earlier to create a safer and more appealing patient experience.
Cybersecurity
Protecting the power of connected systems.
Connected device systems are evolving to have more complex integrations between devices, mobile, cloud and 3rd party systems. Orthogonal recognizes that more powerful integrations that deliver greater value can also carry higher risk. Cybersecurity is an ongoing process that is woven into our product development lifecycle, and is compliant with the FDA’s pre- and post-market Cybersecurity guidance for medical devices.

Software Segregation
IEC 62304 compliance leads to faster evolution.
Software segregation isn’t only a good design principle – it makes risk mitigation easier and more maintainable in a complex system. Orthogonal applies modularity and software segregation techniques as per IEC 62304 to mitigate the risk of cascading failures and allow for easier product maintenance and evolution.
Risk Management & Interoperability
Enabling safe interactions within complex environments.
As devices become more interconnected, interoperability of SaMD and connected medical device systems becomes more important. Orthogonal designs for interoperability, building software development kits (SDKs) tailored for integration. We perform risk analysis and risk mitigation for modules and SDKs, including interoperability risks. We document these residual risks so that interoperators can mitigate them as part of their systems. Using these same techniques, we build out reusable platform components for easy integration into medical devices.

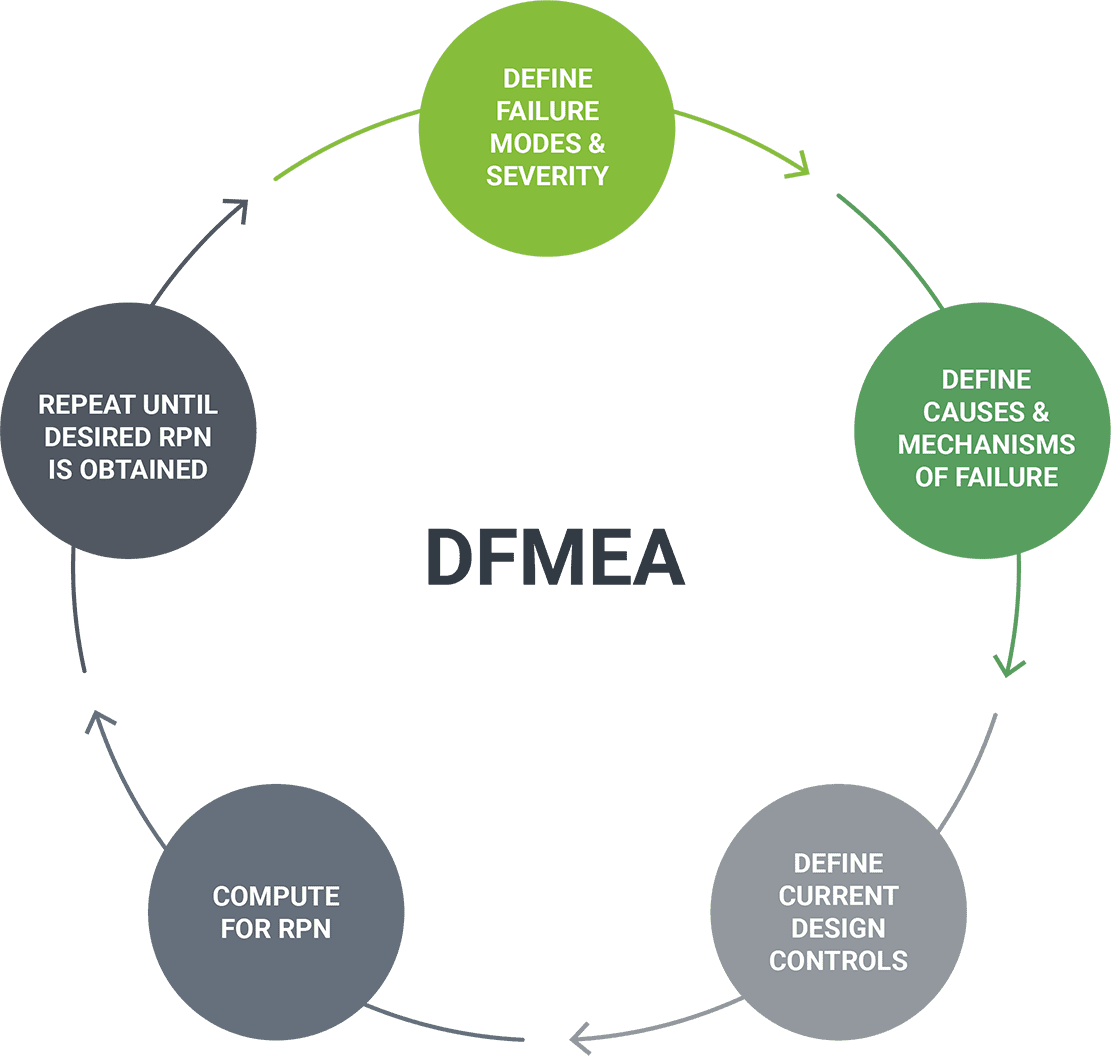
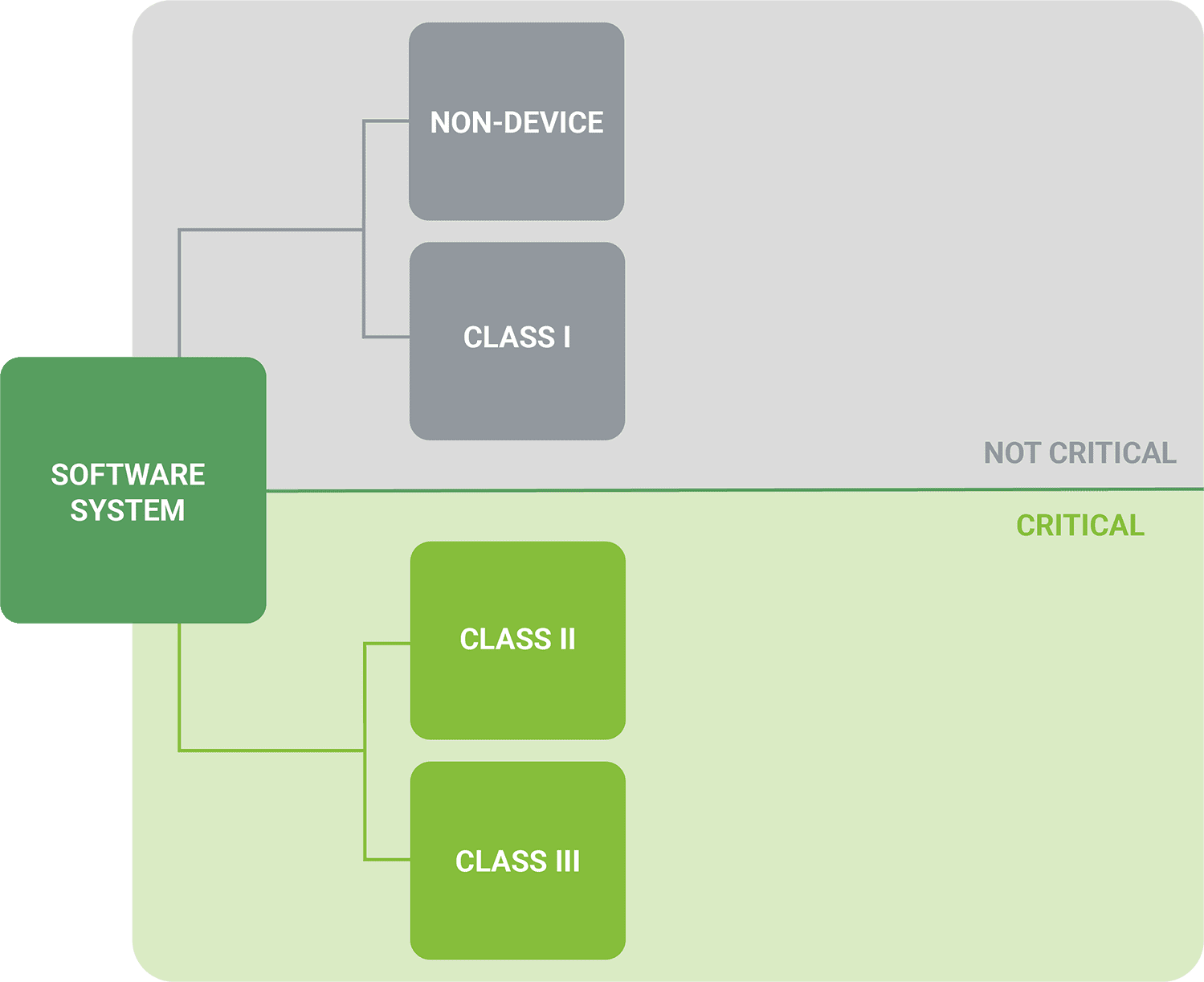
Rightsizing Risk Analysis
Keeping complexity manageable.
Orthogonal performs risk analysis techniques that scale to the software level of concern and software safety classification. These include hazard analysis, fault-tree analysis, design failure mode and effect analysis (DFMEA) and use failure mode and effect analysis (Use FMEA). Incorporating these techniques throughout the product lifecycle allows us to catch issues faster and earlier, thus helping you avoid recall during the FDA approval process.