Case Study
EBT: External Nerve Stimulation for Overactive Bladder
Executive Summary
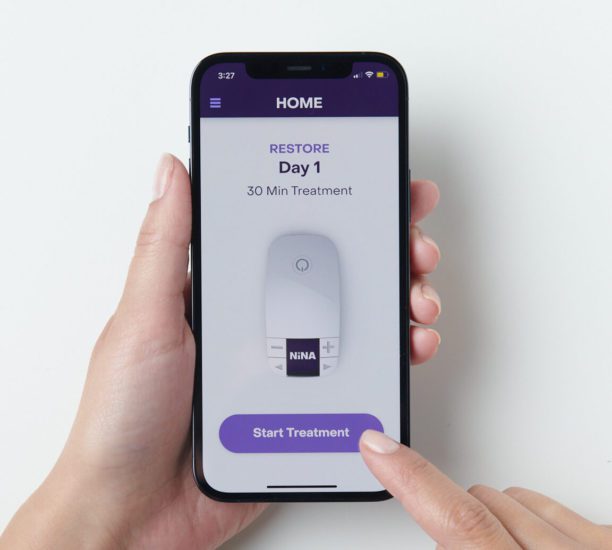
Orthogonal worked with EBT Medical, a venture-backed startup developing paradigm-shifting neuromodulation technologies focused on treating pelvic health disorders via EBT’s novel, patented Saphenous nerve target, to create a mobile app for NiNA™ (Noninvasive Neuromodulation Assistant), an external neuromodulation device that is currently in development. We used our Quality Agile process to build out iOS and Android mobile apps (branded NiNA Care™) that connect to the NiNA device via Bluetooth Low Energy (BLE) and established cloud services to support the NiNA system. The NiNA Care mobile app guides users in how to properly use the device and deliver the correct neurostimulation therapy, allows them to initiate therapy and change therapy settings, and offers treatment reminders and content that maximizes patient engagement.
Scope
EBT Medical, spun out of the University of Toronto, is a venture-backed startup and the developer of the NiNA™ system. NiNA is an external neuromodulation device that targets the Saphenous nerve to treat Overactive Bladder (OAB). Unlike implantable neuromodulation devices, NiNA is a wearable system that rests on the outside of the patient’s upper calf and delivers noninvasive Saphenous Neuromodulation (nSAFN) therapy in 30-minute daily sessions.[1] Once it achieves market authorization in the U.S., Europe and other countries, NiNA will offer the potential of a safe and effective treatment, empowering patients to receive effective care for OAB in the comfort of their own home.

Challenge

EBT sought Orthogonal’s help in creating a companion mobile app and cloud backend for NiNA. To meet the product requirements, the NiNA Care app needed to reliably connect via BLE with the NiNA device, initiate treatment, change treatment settings, have the ability to capture data about the device and its usage, sync that information with the cloud and enable smooth over the air (OTA) updates for device firmware. The app must provide on-demand videos to guide patients in the proper use of the device, including positioning and stimulation control to deliver the correct therapy treatment. As a “Bring Your Own Device” app, it had to run smoothly on a wide gamut of iOS and Android smartphones and operating systems. The app also needed to meet the specifications of EBT’s upcoming multi-arm clinical trial.
Orthogonal has worked with a growing number of neuromodulation companies to build ecosystems of mobile apps and cloud computing around their externally applied stimulators and active implantables. Our prior success in this clinical domain and our Agile methodology made us the ideal partners to help EBT build out NiNA’s companion app and intelligent ecosystem.
Approach
Orthogonal employed Agile methods, test-driven development (TDD) and behavior-driven design (BDD) to build out the application as well as the requirements and verification protocol. Complete risk assessment and mitigation was finalized on the NiNA Care app in compliance with medical device requirements. The services we provided include:
- Agile collaboration with EBT and their hardware partner to enable parallel development of hardware, firmware, mobile and cloud.
- Cross-platform development of iOS and Android apps with Flutter.
- Cloud backend on Amazon Web Services using AWS microservices.
- 1:1 Bluetooth bonding between device and app for added security.
- Graceful handling of Bluetooth edge cases with guidance for the user to navigate the technology Custom over-the-air update protocol for firmware.
- System architected to easily enable deployment of multiple versions of the app, and transition of patients from one version to the other, in support of a multi-arm clinical trial.
Orthogonal’s emphasis on UX and human factors means we design software with the end user in mind. To better engage with the target audience, additional guided use tutorials were added to the NiNA Care app. A brief questionnaire that provides feedback based on the user’s input helps make sure their device is placed correctly. The app also guides users on how to adjust the stimulation based on their unique physiology, so that the correct amount of neuromodulation is applied in the right location. Furthermore, the app captures data about the device usage and displays device history to users, encouraging their interaction with and adherence to the therapy. It allows patients to better treat and manage their condition and gives them a sense of control over their symptoms.

Result
Ultimately, Orthogonal delivered a system that was well-tested, smartly designed and positioned EBT to pursue successful commercial development for the U.S. and other markets.
“Orthogonal knew how to learn from users, so we could rapidly incorporate insights picked up during the project,” says Keith Carlson, CEO of EBT. “We jointly embraced that agility because it resulted in the best product for users.”
“We chose Orthogonal because they know how to develop great medical device software under design controls. Their prior work with neuromodulation devices made us confident in our choice. They proved to be not just a vendor but a true collaborator. When issues popped up, the team jumped right on a call and worked through it. They were flexible and focused on getting the right stuff done,” says Mike Labbe, VP of R&D.
Reference 1. THE SCIENCE – EBT Medical & NiNA. Ebtmedical.com. https://ebtmedical.com/the-science/. Published 2021. Accessed May 20, 2022.