Case Study
Continuous ECG Monitoring with Nanosensors
Scope
Millions of individuals suffer from chronic, debilitating diseases that require continuous monitoring. Until recently, no extant monitoring application afforded patients complete freedom of movement and attendant peace of mind.
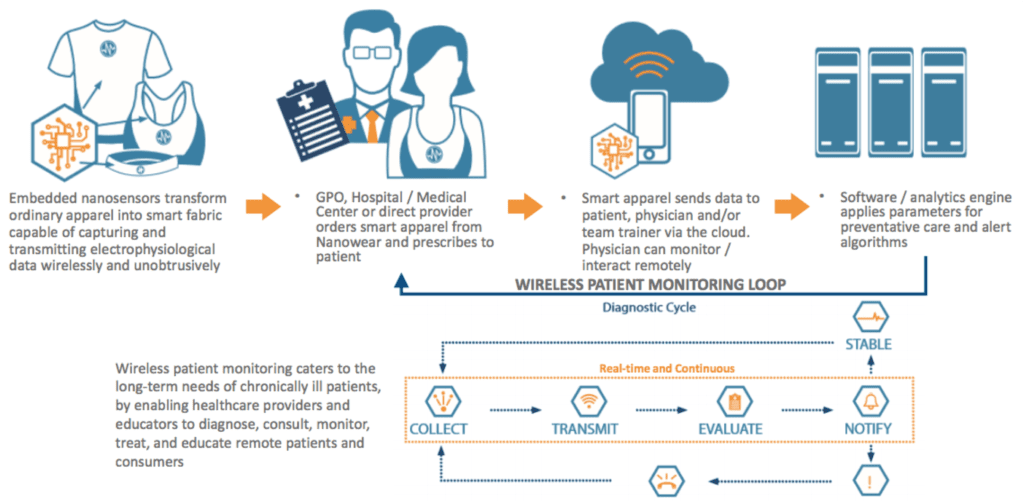
Medical device startup Nanowear changed all of that. Nanowear envisioned a groundbreaking flagship system for remote monitoring of cardiac patients, using a network of proprietary biosensors embedded within everyday undergarments such as brassieres and t-shirts. Not only is remote, continuous monitoring now available, it simultaneously provides medical practitioners with a flexible, cost-effective platform to make informed decisions. And that’s a win/win.
Challenge
When Nanowear’s new product, SimplECG TM, was in its early stages of design, the company sought software companies to work concurrently with them in its development: although Nanowear is a medical device firm, software was not a part of their core. CEO Venk Varadan called on Orthogonal, the Chicago-based medical device software firm at the top of their list of options. Beginning with Orthogonal’s Inception, they moved into an aggressive parallel development schedule for both hardware and software.
It was soon discovered that the original architecture called for Bluetooth Low-Energy (BLE) connectivity between the device and mobile application, and iOS, the initial platform of choice.
However, using BLE would have required active initiation of data transfer by the patient and would have necessitated “waking up” the application from the background remotely. (Currently, only foreground processing works with BLE.)
Approach
Therefore, Bluetooth Classic was chosen instead. From there, Orthogonal collaborated with Nanowear to apply Orthogonal’s full lifecycle product development approach to developing software that captures continuous monitoring of data from a smart-shirt, 5-lead ECG and a smart-bra, 3-lead ECG. The device was architected to use Bluetooth Classic in order to allow for device-initiated data synchronization. Additionally, keeping to the iOS platform, developers enrolled in the Apple MFi program.
Orthogonal then applied interface control processes and a lightweight testing and simulation framework, enabling rapid parallel development of device and software. This enabled faster build-test-fix cycles for both hardware and software by surfacing system level issues early.
The first generation application was developed within a 4-month period and pilot trials were initiated.
Result
The successful result was a simple wearable system that generates continuous, time-stamped, patient-generated symptom information correlated with waveform data. The network provides back-end processing on Amazon Web Services (AWS) with reporting and analysis interfaces of ECG, heart rate and respiratory rate data from the garment via web portal, and further integrates with electro- physiologists and diagnostic cardiology management systems.
SimplECG™, including the software developed by Orthogonal, received FDA clearance as a Class II device December 1, 2016.
Co-founder and CEO Venk Varadan stated, “This is a big milestone for our young company. The FDA’s decision … provides accreditation of the company’s one-of-a-kind, cloth-based sensor technology as medical-grade. This is the first step and foundation of what we believe to be an extensive array of applications for our nanosensor technology – including numerous other electrical, biometric and biochemical signals that can be measured directly from the skin without conductive gels, adhesives or skin preparation.”
Orthogonal’s CEO, Bernhard Kappe, added: “It is always exciting to see the medical validation of a startup vision. Nanowear’s team never wavered from their goal to create a best-in-class diagnostic tool based on unobtrusive undergarments. We are proud to have been a partner in the process.”