Cloud for Medical Devices
Leverage the Cloud’s Power While Ensuring Regulatory Compliance
Cloud computing lends tremendous power and flexibility to medical device companies, but also presents challenges to traditionally held design control paradigms. As leading developers of cloud-based medical device software and co-writers of industry leading cloud guidance, Orthogonal is uniquely positioned to help companies take advantage of the cloud in a compliant manner.

Benefits of Cloud
On-demand scaling of medical device software, algorithms and support infrastructure.
Cloud computing enables software to easily and quickly scale based on real-time demand. Fierce competition among cloud providers leads to services that are continuously updated and improved, making the cloud more reliable, robust and secure than what an individual company could build on their own. With the right approach, one can move medical device software that may otherwise live in a physical device or an internally maintained data farm onto cloud infrastructure to take advantage of the cloud’s overwhelming strengths.
Challenges of Cloud
Highly regulated software meets a continuously evolving environment.
Placing medical device software on the cloud involves relinquishing control over the environment it runs in. As the underlying cloud infrastructure is continuously changing, maintaining software assurance and validation for medical device software becomes a challenge, particularly in the context of traditional software validation paradigms. Orthogonal is at the forefront of reshaping this paradigm to help medical device companies overcome these challenges.

Our Cloud Leadership
Driving alignment between the FDA, medical device industry and cloud service providers.
As part of our effort to redefine software validation, Orthogonal has co-chaired the AAMI Working Group on cloud computing, a collaboration between the FDA, industry leaders in medical devices such as Medtronic, Philips and Abbott, and the major cloud vendors Amazon, Microsoft and Google. Our goal is to create clear guidance enabling medical device companies to effectively utilize the cloud while aligning with the FDA. The group’s initial Consensus Report is available to read, while our final guidance, TIR115, is expected to be released in 2024.

Clouds We Work On
Leveraging our familiarity with Amazon Web Services, Microsoft Azure and Google Cloud Platform.
Orthogonal has experience working with the three major cloud providers: Amazon Web Services (AWS), Microsoft Azure and Google Cloud Platform (GCP). Each provider has its advantages and disadvantages. We’ll help you in selecting the right platform that suits the unique requirements of your medical device software solution.

Ecosystem Development
Supporting your device with our end-to-end product development expertise.
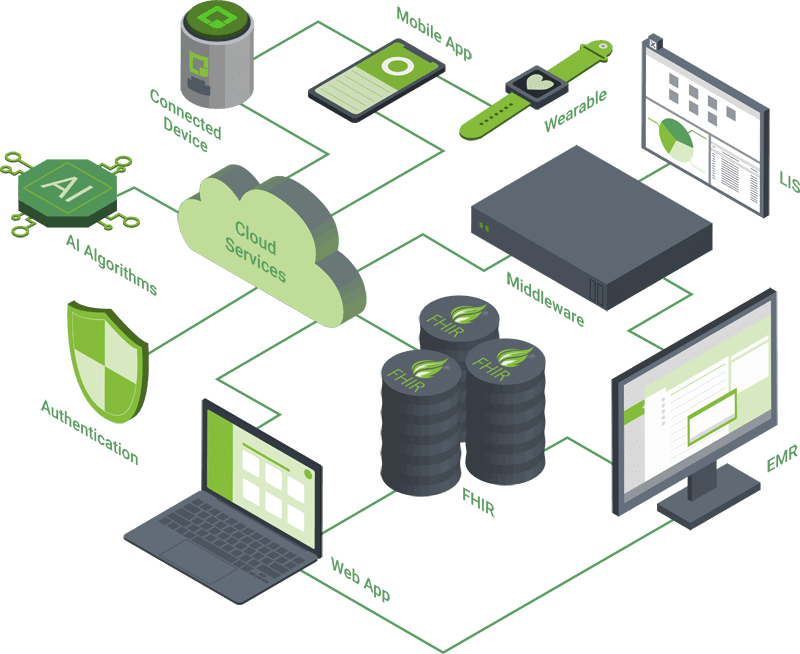
Orthogonal builds scalable medical device ecosystems that encompass device connectivity (Bluetooth, cellular, etc.), mobile and web applications, AI algorithms and cloud services supporting hundreds of thousands of devices and users. Our expertise extends beyond knowledge of each technology and into how they can be architected to work together in a compliant manner. We’ll help your company create an end-to-end ecosystem that ensures regulatory compliance for your medical device in our interconnected world.
Start Your Cloud Conversation
Optimize your medical device software infrastructure to achieve cloud success.
Setting up medical device software or Software as a Medical Device (SaMD) on the cloud reliably and securely requires expert partners. Orthogonal can help you address the quality aspects of software assurance for the cloud. We’ll lead a discussion on the architecture of your cloud services to support your medical device software or SaMD, as well as build out scalable software functionality on the cloud tailored to your project’s needs.
