
Article
Building Interoperability in SaMD: Tools and Best Practices
I’m stoked to work with Elisabeth George. She’s incredibly respected in the MedTech industry, with almost 30 years of leadership at Philips in a unique, globally focused role, and is the recipient of two different industry lifetime achievement awards (the NEMA Röntgen Award and the ANSI George S. Wham Leadership Medal).
I met Elisabeth through her colleague Pat Baird and we hit it off immediately. She’s a great person, a dedicated, passionate leader and a servant to the industry and her staff. Now that we live near each other in South Florida, she’s my favorite lunch date. So when she left Philips, my first thought was, “Hey, Orthogonal has got to find a way to collaborate with her.”
I couldn’t be happier to announce that Elisabeth will join Orthogonal under the title of Regulatory Fellow. She’ll be sharing timely and insightful thought leadership through webinars and blogs, representing Orthogonal in several AdvaMed working groups that she’s been involved with for years, and lending her regulatory expertise to our clients. This blog is the first of many you’ll be seeing from the dynamic duo of Orthogonal and Elisabeth!
_________________________________________________________________________
2022 was another busy year for the FDA’s Center for Device and Radiological Health (CDRH). The CDRH’s annual report, issued on January 27th of 2023, helps the MedTech industry understand what the feds accomplished and why. I’ll be going over the report and giving my insights into trends and results.
Since 2020, it’s been all hands on deck at the FDA to combat the COVID-19 pandemic. This has caused some medical device submission reviews to be delayed. I’ve heard that the FDA even told some companies, “We’re too busy. Come back in a year.” As the pandemic moves onto its next phase, FDA CDRH will continue to work through their medical device submissions backlog.
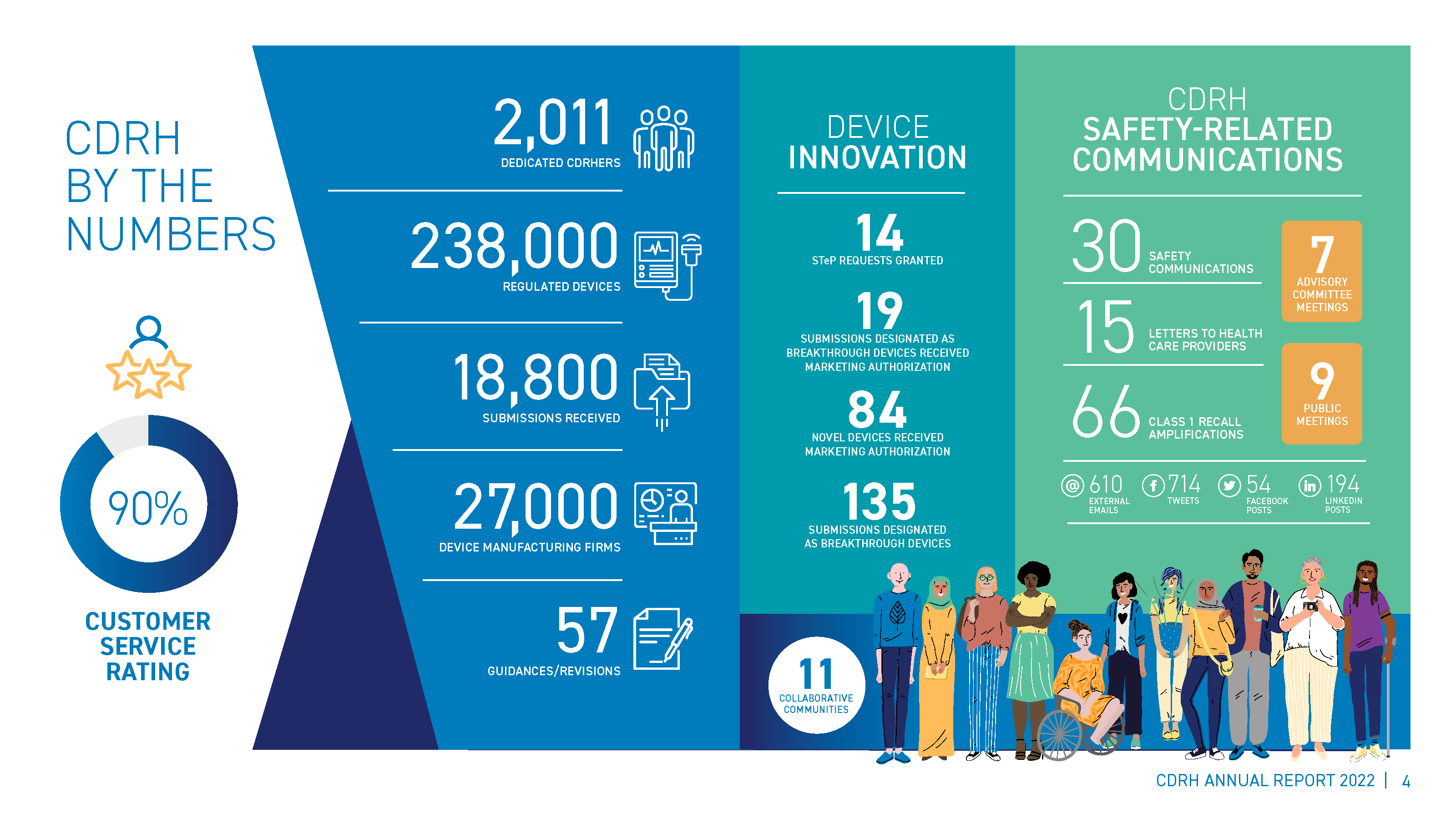
In 2022, CDRH took a number of steps to tackle unique challenges that technology and innovation are bringing to medical device regulations. The FDA User Fee Reauthorization Act of 2022 included a reauthorization of the Medical Device User Fees Amendment (MDUFA V) for five more years, an increase of medical device establishment user fees, the launch of new pilot programs and the shifting of certain programs from pilot to permanent. Relevant to this audience is the Accreditation Scheme for Conformity Assessment, a program in which medical device products tested in FDA-accredited labs need only submit a single declaration of conformity rather than a 100-page test report, significantly cutting down on review time.
One of the highlights of 2022 was the development and launch of the Total Product Life Cycle Advisory Program (TAP) pilot. This program aims to improve aspects of medical device development by providing more frequent and earlier communications between the CDRH, stakeholders and payers, providers and patients. It sounds like a great idea, but there’s a lot we don’t know yet about the program. Time will tell if it can complete its mission of supporting novel concepts through rapid development.
Device innovation was another area of focus, with the CDRH issuing an impressive number of novel market authorization and breakthrough designations in 2022. The report touted the Premarket Approval, Humanitarian Device Exemption, De Novo and Emergency Use Authorization pathways. Having options is great, but it can be hard for manufacturers in our industry to narrow down which one is best for their products – especially for small medical device companies (80% have less than 50 employees) who do not submit often or may be working on their very first submission. It’s best not to ask what to do, but rather justify your choice so that you don’t end up going with the more expensive option.
Over the past few years, the FDA’s level of interest in Digital Health has skyrocketed. After dabbling in Software as a Medical Device (SaMD) and Software in a Medical Device (SiMD), 2022 was focused on Artificial Intelligence (AI) and Machine Learning (ML). CDRH authorized more than 500 AI/ML enabled devices, and published final and draft guidance documents in Clinical Decision Support Software and Cybersecurity respectively. As many of these areas are horizontal in nature, the FDA is heavily engaged in standards and conformity development globally, learning and leveraging best practices from non-medical industries.
CDRH also recognized that they must engage across the Total Product Life Cycle (TPLC), in both pre-market and post-market methods, to support their stakeholders. In 2022 they focused on Real World Evidence (RWE) and Real World Data (RWD). RWE/RWD draws from across an entire ecosystem – not just a manufacturer collecting feedback as part of their QMS requirements or users submitting complaints. This information can help stakeholders understand how their products are being used, as well as aid in identifying vulnerabilities and adverse effects.
Even with all of the hard work the CDRH has done over the past 40+ years, there’s still a lot more to do. There are companies unclear if they’re developing, manufacturing or servicing a medical device; innovation is continuous and moving at the speed of light; mistakes occur that may result in recalls. CDRH needs to listen to and learn from stakeholders, so that together we can innovate our solutions, services and methods. We don’t want to be shut out with a blatant “NO!” We want to be an equal part of the discussion on moving forward.
Want more insights from Elisabeth? Watch our short interview with her.
Elisabeth George is an experienced leader, business executive and consultant committed to shaping and leading global organizations in continuous improvement and innovative ways of using standards and regulations, not only for compliance but also for supporting customer value. She frequently speaks at Industry and Standards Conferences where stakeholders from Regulators, Industry and Users (Clinicians & Patients) participate. She is a recent recipient of the NEMA Röntgen Award and the ANSI George S. Wham Leadership Medal recognizing her leadership and drive. She is committed to making an impact through her skills as a leader, her experience in Medical Device Regulations and Standards and her desire to be a learning partner in delivering positive business outcomes. She is presently serving on the ANSI Board of Directors.
Elisabeth has held senior leadership roles for more than 30 years, joining Haemonetics in 1989 as a Director of Quality and Regulatory to Sr. Director and Vice President of Quality and Regulatory positions with Hewlett Packard, Agilent and Philips.
At Philips she held the positions of Vice President of Quality, Regulatory, Sustainability and Product Security responsibility for two of the major product lines. She also held the position of Vice President/Head of Global Regulations and Standards and represented Philips in trade associations, regulatory body advisory panels and standards development organizations such as AdvaMed, AAMI, ANSI, NEMA, IMDRF and FDA Advisory Panels.
Elisabeth holds a Bachelors in Science, Biomedical Engineering from Boston University and a Masters Certificate in Engineering Management from Northeastern University.
Related Posts

Article
Building Interoperability in SaMD: Tools and Best Practices
Article
Leveraging AI/ML in SaMD Development: Benefits and Challenges
Article
ISO 13485 Compliance Checklist for SaMD Development
Article
How to Conduct Post-Market Surveillance for SaMD