Article
Regulatory Innovation in SaMD: How Global Standards Are Evolving
This blog contains Part 2 of the Orthogonal and MedSec white paper titled “Making the Significant Insignificant: Implementing a Predetermined Change Control Plan (PCCP) for Class II SaMD Products Beyond AI/ML.” The following are links to each part of this white paper:
– – – – –
The PCCP is a new mechanism that allows the FDA to exempt certain planned changes from the premarket notification or the premarket approval submission requirements if those changes implemented in the future are consistent with the PCCP authorized by the FDA. Prior to FDORA, such changes would otherwise require 510(k) or PMA submissions.
Therefore, it is not necessary for manufacturers to include any planned change that would be assessed as a document-to-file under the existing regulations and FDA guidance, as such minor changes do not require 510(k)s, PMA or PMA supplements without a PCCP.
Specifically for non-exempted Class II SaMD software, planned changes that would lead to a “Document” decision in the same decision-making flowchart from the 510(k) software modification guidance (illustrated below, the same flowchart as previously appeared in Section 2.2) are not suitable for the PCCP. Manufacturers can simply go through the change control process, assess the impact of the change, and properly decide on a document-to-file regulatory pathway before implementing the change, without a need for such future changes to be included in the PCCP for pre-authorized by the FDA.
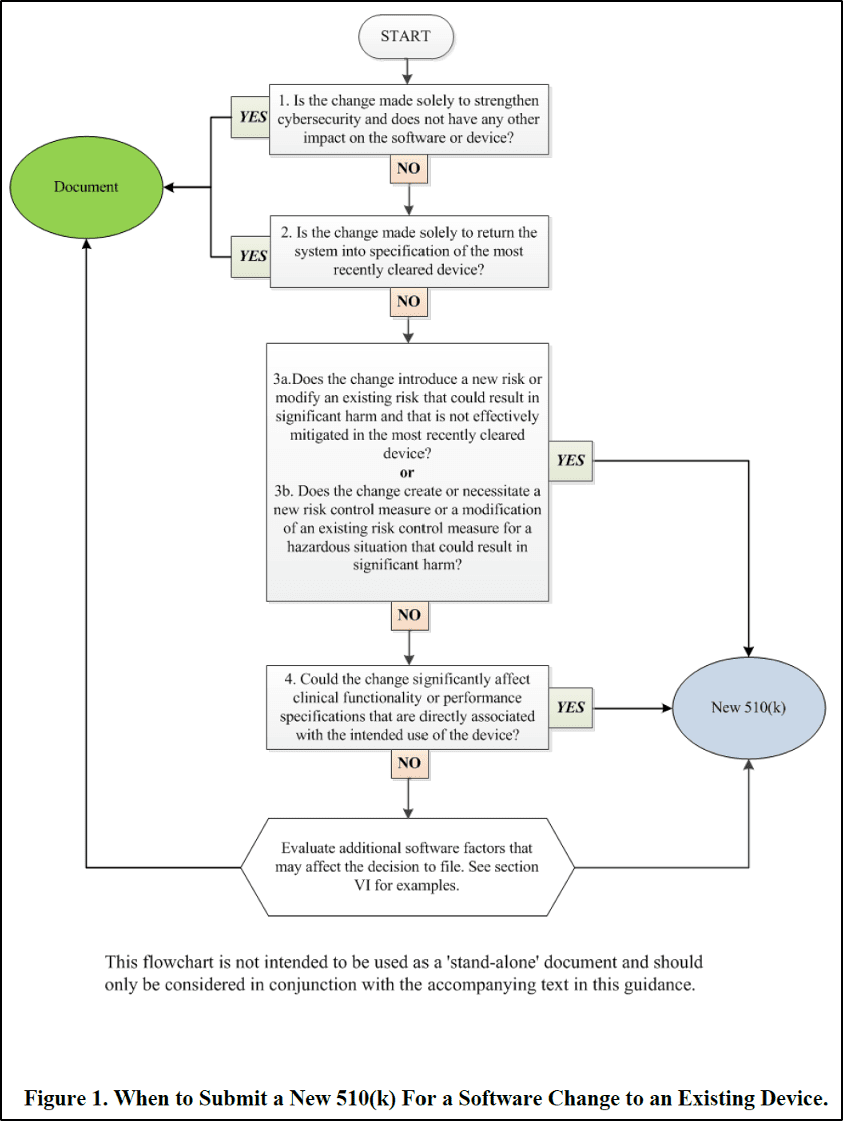
For the same reason, suitable changes to be included in a PCCP are the type of future changes that would fit in one of the decision boxes in the above flowchart and result in the decision to file a “New 510(k).”
Since FDORA does not change the requirements for 510(k) devices, the FDA expects that any changes included in a PCCP for a 510(k) device must be limited to modifications that do not affect the device’s safety or effectiveness. Software with these modifications must also be substantially equivalent to the last cleared version of the software or the last granted version through the de novo classification process. We offer the following suggestions to both device manufactures and the FDA about how to evaluate which changes might be a good fit for this new regulatory state, and which might be poor fits.
Section VI of the 510(k) software modification guidance includes the change categories listed below. The FDA presented these as “additional factors to consider” without indicating the appropriateness of a new 510(k) submission, since the potential impacts from these categories of software changes vary greatly and are difficult to be summarized in a flowchart format. If your planned software changes fit into these categories, they could be good candidates for PCCP changes as long as you describe in detail in the PCCP your change impact assessment process, performance criteria, etc.
– Infrastructure
– Architecture
– Core algorithm
– Clarification of requirements
– Cosmetic changes
– Reengineering and refactoring
Next, we suggest that manufacturers focus on decision boxes #3 and #4 in the Figure 1 flowchart above for evaluating the appropriateness of potential candidate changes for inclusion in a PCCP. Specifically, manufacturers should carefully review the 510(k) software modification guidance’s Appendix A software modification examples, especially software changes that lead to a “New 510(k)” based on flowchart questions 3a, 3b and 4. Not all such changes are good candidates for the PCCP, but some could be with an appropriate control plan presented in the PCCP and implemented by the manufacturer.
A SaMD software change can lead to changes in software labeling, performance specifications, or wireless communications, for example. To properly decide whether or not a new 510(k) is required for a proposed software change, in addition to using the 510(k) software modification guidance, a manufacturer should also refer to the 510(k) device modification guidance (i.e., Guidance for Industry and Food and Drug Administration Staff, Deciding When to Submit a 510(k) for a Change to an Existing Device, October 23, 2017).
Similarly, we suggest that a manufacturer carefully review Flowchart A (labeling changes) and Flowchart B (technology, engineering and performance changes) in the 510(k) device modification guidance and examples of relevant changes (in Appendix A) that currently lead to “New 510(k)” decisions to determine whether there are appropriate change types that can be included in the SaMD PCCP.
It’s important to keep in mind that a PCCP is a request to FDA to pre-authorize certain future changes to a medical device without lowering the standard for safety, effectiveness, or substantial equivalency. Making PCCP determinations is not something that we expect FDA reviewers to take lightly.
To improve the chances of FDA authorization for a PCCP, in addition to selecting appropriate changes or change types to be included in the PCCP, it is crucial to provide a comprehensive and transparent explanation of the change control process in the PCCP (refer back to Section 2.1 for what change control processes should cover). The PCCP should demonstrate that the changes will be well-understood, well-controlled, thoroughly tested and properly implemented to ensure safety and effectiveness. We suggest that an effective predetermined change control plan for non-exempt Class II SaMDs could include the following key components:
1. Software description
The PCCP could include a detailed description of the product, including software functions, features and key UI designs, architecture charts, interface specifications among different modules if the software uses a modular design, and technologies used (e.g., computer vision, machine learning (ML) or deep learning (DL)).
2. Types of planned future changes covered by the PCCP
The PCCP could specify the anticipated or planned future changes that are covered (i.e., in scope) by the proposed PCCP, i.e., future changes for which the manufacturer is seeking pre-authorization from the FDA through the PCCP.
Equally important, the manufacturer could indicate future changes that are explicitly excluded (i.e., out of scope) for the proposed PCCP.
Detailing specific in-scope and out-of-scope change examples can help the FDA understand the scope and the boundaries of the proposed PCCP.
We also note that based on past experience, it is not uncommon for the out-of-scope list to be longer than the in-scope list.
3. Change control process description
The PCCP could describe the manufacturer’s change control process in sufficient detail, including which functional groups are involved, impact assessment including the risk assessment process, how the proper level of verification and/or validation testing is decided, how to determine whether a proposed change is or is not consistent with the FDA-authorized PCCP, software change deployment and postmarket performance monitoring and control plan, etc.
1. A key element of the change control process for PCCP is the decision-making process and criteria for deciding whether each proposed future change is within the scope and consistent of the PCCP. Such decision-making criteria may include clinical or functional performance criteria. This adds to the process described in Section 2.1, but Step 4 assessing whether a new 510(k) is required also takes into consideration PCCP.
4. Labeling
The PCCP could describe whether, and under what circumstances, new labeling is required for safe and effective use of the modified software and how the new labeling is presented to the users. For example, such circumstances could be when a change requires a new warning, affects instructions for use, or results in significant user interface changes. The updated labeling should be presented in a clear and transparent way for users to understand the changes and the potential impact.
5. Post-deployment control plan
The PCCP could include the plan for real-world performance monitoring post-deployment, and the criteria and process of reverting the change and/or notifying users if the modified SaMD does not function as intended.
Question 1: What is an appropriate level of abstraction for the proposed changes included in the PCCP?
Answer 1: There has been significant debate around whether the proposed changes in the PCCP should be specific individual future changes, or specific types of future changes. Describing a planned individual software change in detail can be a challenging task, and the actual change implemented will likely deviate from the plan to some extent. Therefore, it might be more effective to include specific types of changes in the PCCP, rather than specific individual changes. For example, “Software modifications to update dashboard layout without content change and/or to adjust the number of images that can be viewed simultaneously on the dashboard for better user experience” would be a type of change, whereas as “Software modification to increase the number of simultaneously displayed images from two to five, and to move displayed images to lower-right corner on the dashboard” would be a specific individual change.
This approach would allow for more flexibility in the decision-making process for manufacturers, while still maintaining the robustness of the PCCP change control process. By describing specific types of future changes and the decision-making process, the PCCP can accommodate changes that may not have been anticipated during the planning phase but for which the manufacturers have sufficient oversight to demonstrate that the FDA could entrust them with the discretion offered to them by a PCCP.
Question 2: Does the PCCP cover non-exempt Class II and Class III device constituents of combination products?
Answer 2: This question is currently pending clarification from the FDA. It is possible that the general principles and required process for the PCCP may apply to software device constituent parts of combination products. It is important to note that combination products are subject to complex regulatory requirements, and that PCCPs may need to be adapted to address the specific needs and risks of each product.
Question 3: Are there qualification requirements for the baseline device when requesting the PCCP? For example, can we submit a PCCP for a 510(k) device cleared more than 20 years ago?
Answer 3: Section 3308 of the FDORA does not specify any qualification requirements for the baseline device when requesting the PCCP. However, as a part of the PCCP review process, keep in mind that the FDA may research and use the baseline device’s regulatory and compliance history as one of the factors for its decision-making.
We have included some points that would benefit from clarification regarding the PCCP at the end of this white paper to encourage ongoing discussions among the medical device industry, the FDA, and other stakeholders. We believe that these points are important to consider as the FDA and the medical device industry jointly tread onto the new terrain of implementing PCCPs. Continued discussions and collaboration between all stakeholders will be necessary to address these points and ensure the effectiveness and sustainability of the PCCP over time.
1: In the proposed PCCP submitted to the FDA, does the manufacturer need to provide any supporting evidence to build agency trust?
2: How should the manufacturer and the FDA assess the potential impact of evolving standards and regulations on the already-approved PCCP?
3: How will the FDA verify proper implementation of the PCCP? For example, should the FDA set up a special audit program outside of current for-cause and routine surveillance inspections?
4: Should there be an expiration date for a PCCP? Or should the FDA ask the manufacturer in subsequent premarket submissions after the original PCCP is authorized to provide an updated PCCP or a justification why the existing PCCP will continue to be effective with the modified device?
5: Once approved, can a PCCP be suspended or rescinded by the FDA, under what circumstances?
6: FDORA covered the PCCP but not the software pre-certification program. What are the relationships between the two programs? What is the current status of the software pre-certification program?
– In September 2022, the FDA issued a final report[1] discussing the results of its software precertification program. They concluded that while rapidly evolving medical device software technologies could benefit from the new regulatory framework, such a new paradigm would require federal legislative action. However, with the PCCP approved under Section 3308 of the FDORA, there may be an opportunity to modify the software precertification program and potentially implement it under the PCCP framework. This could potentially involve incorporating some or all five Culture of Quality and Organizational Excellence (CQOE) principles (product quality, patient safety, clinical responsibility, cybersecurity responsibility and proactive culture) as a part of the PCCP.
SaMD products offer unique opportunities for improving patient care, such as the ability to collect vast amounts of postmarket data, address software issues, or add new features quickly in response to real-world performance and user feedback. However, the traditional approach to regulating hardware-based medical devices is not well-suited for faster, iterative design and development, and type of validation used for SaMD.
With the approval of Section 3308 under FDORA, the FDA has taken a step towards regulating SaMD in a way that aligns with the unique needs of these products. The PCCP presents a promising framework for SaMD regulation, particularly for AI/ML-based software.
Moving forward, it will be important to continue discussions and collaboration between the FDA, the medical device industry, and other stakeholders to ensure that the PCCP is implemented effectively and that safety and effectiveness of medical devices incorporating software is maintained. Furthermore, it will be crucial to expand the PCCP beyond the initial focus on AI/ML-based software functions to encompass general SaMD products. By doing so, the PCCP regulatory framework can support innovation and provide patients with safe, effective, and high-quality medical device software. Ultimately, the success of the PCCP will depend on the ability of regulators, industry leaders, and stakeholders to work together to address the complex challenges associated with regulating SaMD.
Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD) – Discussion Paper and Request for Feedback. US Food & Drug Administration; 2019. https://www.fda.gov/media/122535/download.
Marketing Submission Recommendations for a Predetermined Change Control Plan for Artificial Intelligence/Machine Learning (AI/ML)-Enabled Device Software Functions – Draft Guidance for Industry and Food and Drug Administration Staff Issued on April 3, 2023. U.S. Food & Drug Administration; 2023. https://www.fda.gov/media/166704/download.
Consolidated Appropriations Act, 2023. Vol SEC. 3308. PREDETERMINED CHANGE CONTROL PLANS FOR DEVICES.; 2022. https://www.congress.gov/bill/117th-congress/house-bill/2617/text.
Deciding When to Submit a 510(K) for a Change to an Existing Device Guidance for Industry and Food and Drug Administration Staff Document. U.S. FOOD & DRUG ADMINISTRATION, Center for Devices and Radiological Health, Center for Biologics Evaluation and Research; 2017. https://www.fda.gov/media/99812/download.
Deciding When to Submit a 510(K) for a Software Change to an Existing Device – Guidance for Industry and Food and Drug Administration Staff. U.S. Food and Drug Administration, Center for Devices and Radiological Health, Center for Biologics Evaluation and Research; 2017. https://www.fda.gov/media/99785/download.
The Software Precertification (Pre-Cert) Pilot Program: Tailored Total Product Lifecycle Approaches and Key Findings. U.S. FOOD & DRUG ADMINISTRATION; 2022. https://www.fda.gov/media/161815/download.
– – – – –
This blog contains Part 2 of the Orthogonal and MedSec white paper titled “Making the Significant Insignificant: Implementing a Predetermined Change Control Plan (PCCP) for Class II SaMD Products Beyond AI/ML.” The following are links to each part of this white paper:
1. https://www.fda.gov/media/161815/download
Related Posts

Article
Regulatory Innovation in SaMD: How Global Standards Are Evolving

Article
Implementing Real-Time Data Analytics in SaMD Solutions

Article
Navigating FDA’s Proposed AI/ML Framework for SaMD

Article
Improving Patient Engagement with SaMD Solutions