What We’ve Worked On
Mobile medical applications that improve patient outcomes.
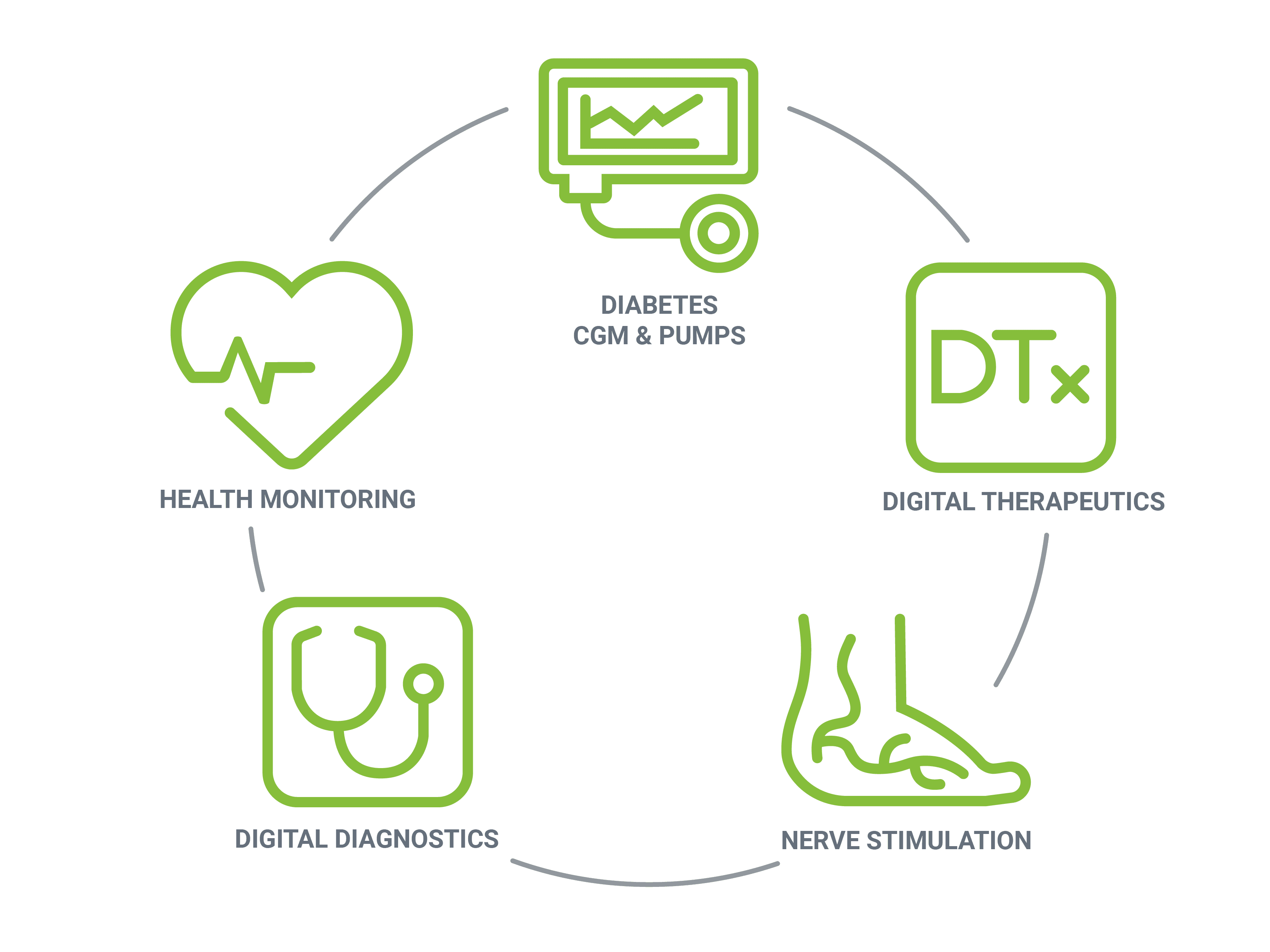
As experienced developers of SaMD and connected device solutions, Orthogonal has brought to life a variety of mobile medical applications covering a wide range of clinical domains – including diabetes, nerve stimulation, health monitoring, Digital Therapeutics (DTx) and digital diagnostics. Our teams deliver high-quality, safe and effective mobile applications that engage and empower patients.
Native iOS & Android Development
Delivering high-performance apps for higher-risk connected devices.
Higher-risk connected devices, like pumps, implantables and sensors, need apps that deliver on performance and reliability. Native development in Swift (for iOS) and Kotlin (for Android) is ideal for highly interactive applications that leverage native smartphone sensors and Bluetooth connectivity. Orthogonal has deep experience working in native development, having developed iOS and Android applications to control active implantables, insulin pumps and CGMs.

Flutter & React Native Cross-platform Development
Working flexibly and economically while maintaining performance and scalability.
Orthogonal uses cross-platform development in Flutter (Dart) or React Native (JavaScript) to build both iOS and Android apps on one codebase. This process, ideal for lower-risk applications, saves money on development while maintaining a high level of performance and scalability. Orthogonal has engaged in successful cross-platform development for DTx, external nerve stimulators and device monitoring.
Bluetooth & NFC
Understanding the idiosyncrasies of background communications.
Mobile medical apps rely on Bluetooth and Near-field Communication (NFC) to constantly monitor the connected device, even when the app is running in the background. But because background processing is controlled by iOS and Android operating systems, it takes specialized knowledge to maximize reliability while minimizing risks. Orthogonal has over a decade of experience optimizing Bluetooth and NFC connectivity for reliability and performance. With our large library of edge cases, we can design for, test for and gracefully recover from any issue.
Testing for Patient Smartphone Compatibility
Balancing safety and efficiency with an FDA-compliant approach to testing.
The mobile device market consists of innumerable model and operating system combinations, making it impossible to test every device profile. Orthogonal takes a risk-appropriate strategy for pre-market testing and post-market testing and monitoring. We employ a layered testing approach that includes in-house automated testing on specific device profiles, online device farms, field-level self validation and monitoring using product analytics and crash or performance analytics. Our FDA-complaint approach balances safety with efficiency, ensuring effectiveness across thousands of device profiles.

User Experience & Engagement
Optimizing UX to encourage patient engagement.
Mobile medical applications require a deep understanding of how User Experience (UX) design can contribute to ease of use and reliability. Orthogonal employs UX Design and Human Factors to craft applications that organically engage users through feedback and drive their continued use of the product. We also employ Product Analytics to monitor user behavior to feed optimization of the user experience.
SDK Development
Integrating mobile medical applications into a larger digital ecosystem.
When a connected device is part of a larger digital ecosystem, it opens up new possibilities and revenue channels. Orthogonal has experience developing Software Development Kits (SDKs) for integration of CGMs, point of care diagnostics and Clinical Diagnostics on Consumer Electronics (CD-CE) into telemedicine and hospital-at-home systems in an FDA-compliant manner.

