Quality Systems Engineering
An End-to-End Process Optimized for Agility, Compliance & Quality
A systems engineering approach to quality management focuses on making a measurable difference in quality and safety, while automating design controls documentation to accelerate compliant product releases.
QSE Expertise
Our proven, compliant process for Agile development under design controls.
Orthogonal’s Quality Systems Engineering process is designed to develop Software as a Medical Device (SaMD) and connected medical device systems. We leverage procedures, expertise and experience to efficiently apply ISO 13485, ISO 14971 and IEC 62304 to Agile development under design controls. Techniques like behavior-driven design, software safety classification and software segregation ensure our software is built with regulatory compliance and efficient development in mind.
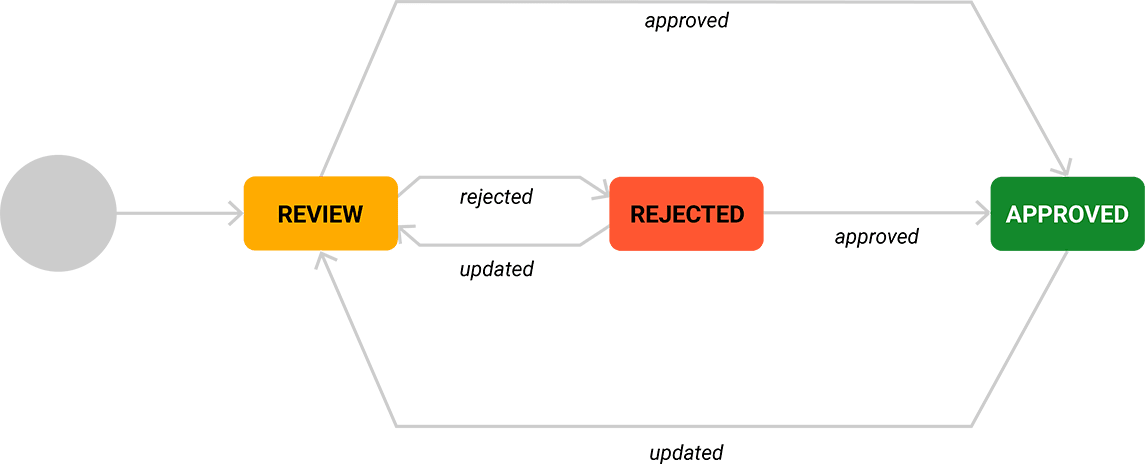
The eQMS Accelerator
ALM, PLM, integrated with familiar tools reduces cost and time by 25-50%.
Development processes are accelerated by our electronic Quality Management System (eQMS) with Application Lifecycle Management (ALM) and Product Lifecycle Management (PLM) features, built on top of Jira and Confluence – tools that many development teams already use effectively. This allows us to rapidly generate, reuse and automate requirements, risk management, specification, verification and traceability documentation. Our eQMS accelerator improves quality while reducing the duration and cost of design controls activities by 25-50% – and enables you to release faster without increasing quality or regulatory concerns.
Our Development Under eQMS
Choose us to deliver quality software ahead of time and budget.
Our highly developed Quality Systems Engineering practices and eQMS are integral to Orthogonal-run development projects. With us at the helm of development, we deliver on optimized processes, tooling and automation that save time and improve software releases.

Your Development Under Our eQMS
Use our expert processes to empower your team.
For customers already developing SaMD and connected medical device systems, we apply our market-tested eQMS directly to your work. Both newly launched startups and established medical device companies will reap the benefits – whether you’re looking for more efficient processes tailored to SaMD development, faster releases and product evolution with lower cost, or to set up a framework that lets your development teams focus on development, while Orthogonal focuses on quality and accelerating design controls.

Project Rescue & Remediation
Move forward from regulatory setbacks and quality issues.
If you have hit a pothole on the way to regulatory clearance from the FDA or under EU or UK MDR, or have discovered quality management issues along the way, we can help get you back into compliance. Our team will perform a gap analysis to give you an honest assessment of where you are and a prioritized list of recommendations. Together we’ll determine the most efficient ways to close those gaps, so you can bring existing products under design controls and maintain compliance.
