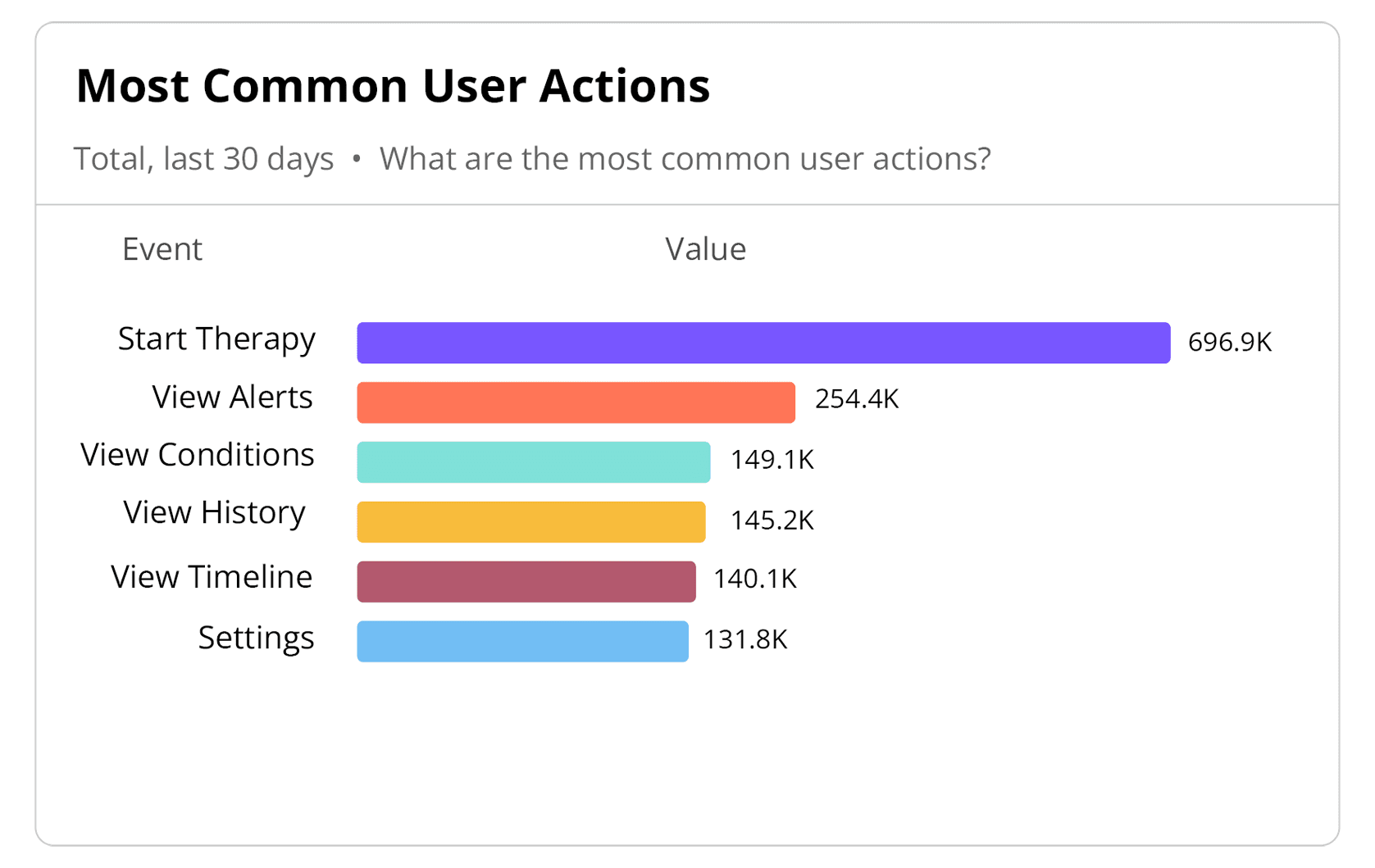
User Experience (UX) Design
Increase patient engagement through
fast feedback loops.
By integrating User Experience (UX) Design, Human Factors (HF) and Product Analytics (PA) in our design process, Orthogonal gets high-quality Software as a Medical Device (SaMD), Digital Therapeutics (DTx) and connected medical device systems to market faster and evolves them faster. With early stage coupling of UX design and HF testing, we implement fast feedback loops that improve the design from both a safety and ease-of-use perspective. We test user performance on tasks and overall appeal of the product iteratively to inform improvements early in the project lifecycle, when it is inexpensive (and possible) to make changes. The key results of this methodology are increased patient engagement and higher effectiveness of healthcare outcomes.
Human Factors (HF)
Small-scale testing delivers high
return on investment.
Orthogonal has found that the impact of early, small and rapid tests (we refer to them as Three User Thursdays) is significant compared to the cost of doing such testing after development. Three User Thursdays set the stage for more formal (and expensive) formative and summative tests. Iterative testing complements our Agile development process. It ensures that the features we develop deliver on the intended use of the device and satisfy potential edge cases.
Product Analytics (PA)
Power your product forward with
real-world user data.
We leverage the power of Product Analytics to ensure feedback keeps flowing when the product reaches a trial or market release. Product Analytics involves instrumenting the medical device software so that key tasks and flows are tracked and the data gathered is displayed in near real-time on dashboards. Insight into how users are really using the product informs design improvements, and helps you ask the right questions during further qualitative testing.