Article
Building Interoperability in SaMD: Tools and Best Practices
Bluetooth technology makes connected medical devices more powerful, more sophisticated and easier to use, helping MedTech companies to improve patient outcomes faster. It allows developers to offload processing power to patients’ smartphones, facilitates the automatic transfer of data from the medical device to a clinician portal or cloud database, and lets patients adjust treatment through their personal smart devices.
With all the benefits Bluetooth provides, it’s no surprise that many FDA-cleared medical devices incorporate it into their systems. What Bluetooth-enabled medical devices received FDA clearance 2023? This updated blog lists all 2023 Bluetooth medical devices that have had their clearance made public and describes how they use Bluetooth.
_________________________________________________________________________
1/11/2023
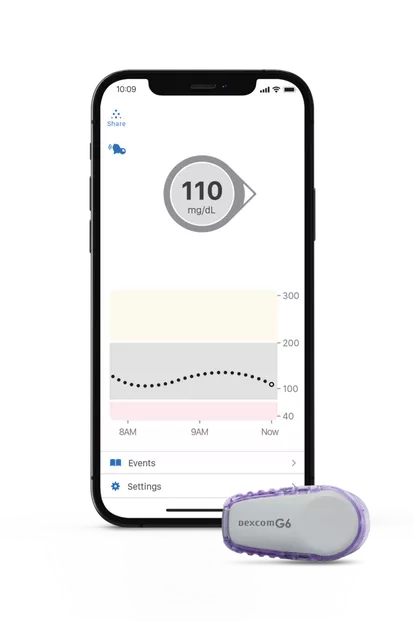
The Dexcom G6 Continuous Glucose Monitoring System (CGM) is an interoperable connected device that measures and displays glucose values for patients with diabetes. It uses BLE to transmit data from the sensor/applicator to a BLE-enabled display device, either a receiver or a mobile application. Dexcom received clearance for their G7 CGM last December. This filing for the previous model includes an alternate receiver to bring it in line with the G7.
1/18/2023

Source: https://exciteosa.com/
eXciteOSA is a removable muscle stimulator that delivers neuromuscular stimulation to the tongue to relieve symptoms of obstructive sleep apnea. It uses Bluetooth to communicate with a smartphone app that controls the device. This device had already received clearance, with a contraindication for people with metal implants in their mouth. Signifier Medical Technologies has since conducted an observational study that demonstrated no significant difference in side effects for people with and without metal dental work. This new filing removes the contraindication.
1/23/2023
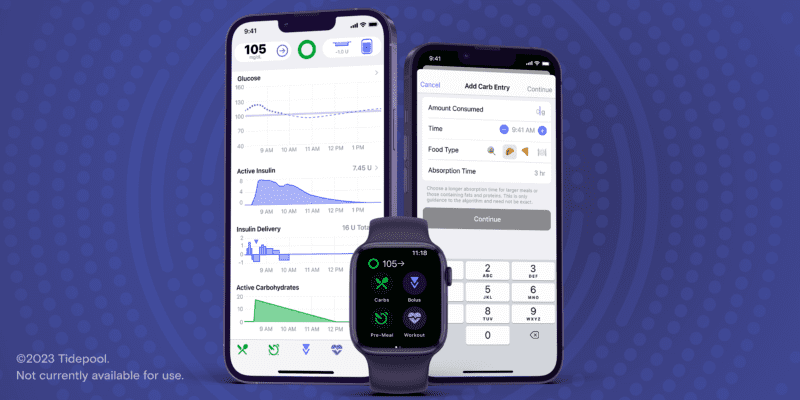
Source: https://www.tidepool.org/blog/tidepool-loop-has-received-fda-clearance
Tidepool Loop is a fully interoperable solution for Type 1 diabetes management. It uses Bluetooth to communicate with any compatible integrated CGM or alternate controller enabled insulin infusion pump.
2/3/2023

Acurable’s AcuPebble Ox100 is a wearable device that records, analyses, displays, exports and stores biophysical parameters that aid in diagnosing sleep apnea. Bluetooth communication is used between the sensor in the device and the AcuPebble companion mobile app.
2/6/2023
Vivify Health is a platform for remote patient monitoring. This clearance is specifically for the platform’s clinician portal. Like many health portals, it allows clinicians to view and manage patient information, schedule video visits and send secure messages, among other things. Patient data can be collected wirelessly over Bluetooth and sent to the portal.
2/8/2023

Hailie Sensor NF0110 is an electronic data capture accessory that remotely monitors patients who use compatible inhalers. This clearance is specifically to add functionality for Teva HFA pressurized metered dose inhalers. Adherium has already received clearance for compatibility with AstraZeneca and GSK inhalers. The Hailie sensor has a Bluetooth interface to wirelessly exchange medication usage data with a paired communications device and compatible mobile software applications.
2/20/2023

Source: https://perifit.co/
Perifit is a perineometer designed to treat stress, mild-moderate urge and mixed urinary incontinence in women, by strengthening the pelvic floor muscles through exercise. The device provides biofeedback to a companion smart phone app over Bluetooth.
3/6/2023
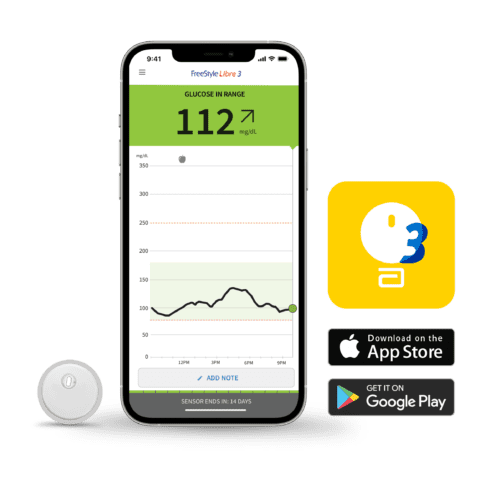
These diabetes monitoring devices from Abbott received new clearance that allows for connectivity with automated insulin delivery (AID) systems. Both systems autonomously communicate with digitally connected devices over Bluetooth.
3/8/2023

From Digital Therapeutics company Respiree, the RS001 is a chest wearable that measures respiration directly. It can be used on patients suffering from cardio-pulmonary diseases such as chronic obstructive pulmonary disease (COPD) and congestive heart failure. It uses Bluetooth to wirelessly transfer patient data to mobile devices.
3/8/2023

Source: https://www.medicaldevice-network.com/news/fda-neuromod-lenire-tinnitus/
Neuromod’s Lenire is a non-invasive treatment device for tinnitus. It combines acoustic and electrical intraoral stimulation to relieve tinnitus pain. The Lenire system includes Bluetooth headphones that connect wirelessly to a handheld controller.
3/9/2023

Source: https://neuro20.com/
The Neuro20 Pro System is a bodysuit that stimulates muscles to improve or facilitate muscle performance. It has a variety of uses, including retraining muscles after an injury and maintaining or increasing range of motion. The device control unit generates and transmits electrical signals to the electrodes in the suit via Bluetooth.
3/8/2023
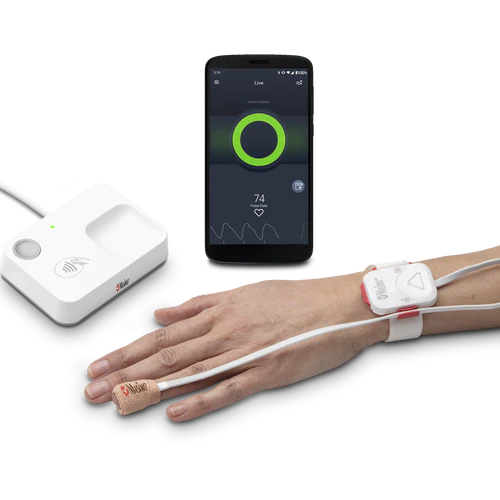
Masimo’s Opioid Halo is a system that detects opioid-induced respiratory depression, the direct cause of death from opioid overdose. It features a tetherless, adhesive fingertip sensor and a reusable Masimo SET pulse oximeter and Bluetooth chip.
4/24/2023

Source: https://www.mobihealthnews.com/news/casana-scores-fda-clearance-health-monitoring-toilet-seat
Your eyes aren’t fooling you. Casana’s medical device is a smart toilet seat that remotely monitors heart rate and oxygen saturation when it is sat on by a patient. It sends health data to providers over Bluetooth, and notifies them when parameters are outside of a set threshold.
4/21/2023

Source: https://www.medtronicdiabetes.com/products/minimed-770g-insulin-pump-system
The Medtronic MiniMed 770G is a closed-loop system for diabetes management. This clearance enables the firmware over-the-air update feature. With this feature, Medtronic can distribute wireless firmware updates over Bluetooth to upgrade patients’ insulin pumps without the need for the pump to be returned. It also allows 770G users to upgrade their pump firmware to the recently approved 780G firmware, which adds new features.
5/5/2023
DyanmiCare’s DCH-002 is a Digital Therapeutic intended to treat alcohol use disorder. It combines a smartphone app with a Bluetooth-connected, police-grade breathalyzer and a reloadable, risk-protective smart debit card. Participants complete random breath tests, and if they test negative, they are awarded a cash reward on their smart debit card (which blocks access to bars, liquor stores and cash withdrawals).

5/15/2023
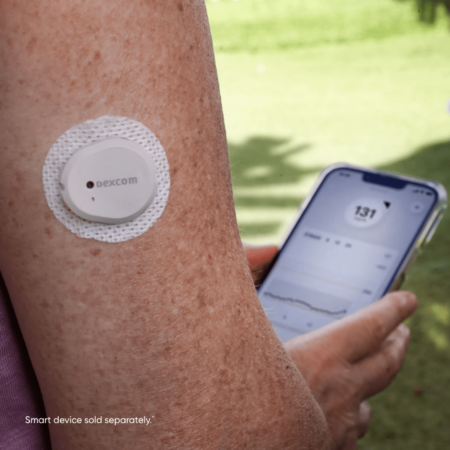
Source: Dexcom
Dexcom’s G7 CGM system received additional clearance to include indication for an adhesive patch for the Bluetooth-enabled wearable monitor backed with non-woven polyurethane, as an alternative to the non-woven polyester backing of the original patch.
5/26/2023
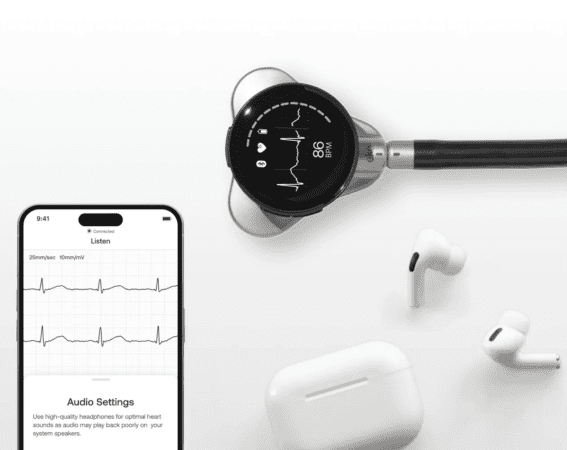
Source: Eko Health
Eko Health’s CORE 500 Digital Stethoscope with a full-color digital screen that displays patient results in real time, as well as leads to perform an electrocardiogram (ECG). The earpieces contain noise-canceling technology. Physicians have the option to connect their own Bluetooth earbuds to the CORE 500. Data captured from the stethoscope is recorded and shared wirelessly via Bluetooth with the Eko mobile app.
6/16/2023

Source: Owlet Baby Care
Owlet Baby Care’s BabySat 3 provides high-quality monitoring of a baby’s vitals in a home environment. The device consists of a pulse oximeter attached to a soft wrap worn around the infant’s foot, which connects via Bluetooth to a base station. The BabySat 3 measures blood oxygenation levels and pulse rate, alerting parents if readings fall outside typical ranges. The data is also shared wirelessly over Bluetooth with a companion mobile app.
July 7, 2023
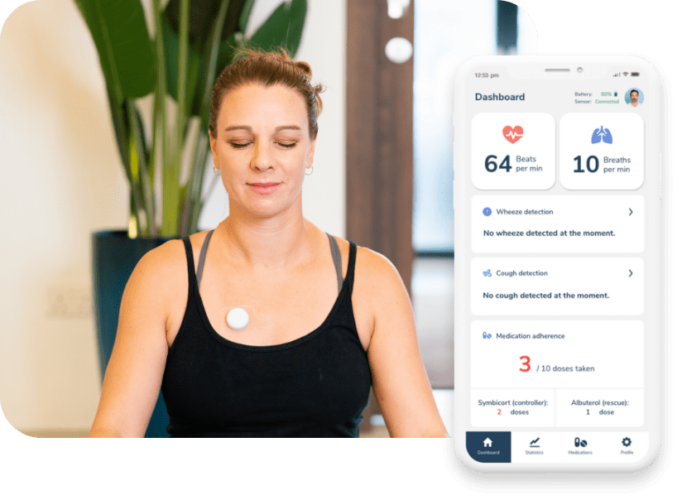
Source: Aevice Health
AeviceMD from Aevice Health remotely monitors a patient’s lung sounds. Patients attach the “wearable stethoscope” sensor to their chests via an adhesive patch. The device listens to the sound of the patient’s lungs while also capturing their heart rate. Sensor data is sent to the docking station via Bluetooth, where the computational power of the device is stored, as well as where the system connects to a cloud platform. Data is shared from the cloud with separate mobile apps for patients and for their clinicians.
July 10, 2023

Source: Tandem
The Tandem Mobi is a small, discrete and interoperable insulin pump for patients with diabetes. It is compatible with Dexcom’s G6 CGM, with support for the G7 system planned. This device is less than half the size of Tandem’s t:slim X2 pump, fitting into the coin pocket of a pair of jeans, and is controlled over Bluetooth by the t:connect mobile app.
Aug 2, 2023

Source: SmartSound
Smartsound’s Skeeper SM-300 is an electronic stethoscope designed for personal healthcare and telemedicine use. It measures the sounds of a patient’s heartbeat, lungs and abdomen, transmitting that information over Bluetooth to a companion smartphone app. Patients can record their own sounds and conveniently share them with a physician via text or email.
August 4, 2023

Source: AION Biosystems
TempShield from AION Biosystems is a wearable thermometer that continuously monitors body temperature and provides alerts when the temperature exceeds a given range. The button-like device connects to a mobile app over Bluetooth, which receives temperature data and processes it using an AI algorithm.
August 10, 2023

Source: ReliOn BGM
The ReliOn line of traditional blood glucose meters from i-SENS is a spinoff of their CareSense line of products, exclusively sold in the U.S. at Walmart. ReliOn Premier BLU measures blood glucose levels using the accompanying test strips, and can share data over Bluetooth with the Glooko diabetes management app.
August 11, 2023
Source: device.report
Otsuka’s Digital Feedback Device-RW is a unique combination of an ingestible sensor placed in a capsule or pill tablet, a physical sensor worn on the torso and a companion mobile and tablet app. Bluetooth enables the transfer of data from the ingestible sensor residing in the patient’s stomach to the wearable sensor on their body, and from there to the app.
The Digital Feedback Device was originally cleared in 2015; this new clearance’s enhanced algorithm allows for the physical patch to be worn on either side of the torso, rather than just on the left side.
August 23, 2023

Source: Withings
The Scan Monitor 2.0, called the Body Scan on Withings’s website, is a smart weight scale with a retractable handle. The scale provides an in-depth assessment of a user’s body, segmenting results for their arms, legs and torso. By gripping the retractable handle, users can initiate a six-lead, 30-second ECG measurement with the Scan Monitor 2.0. Both ECG and body scan results are shared with a companion smartphone app over Bluetooth.
August 29, 2023

Source: circul
The circul pro ring or circul+ sst ring from circul and BodiMetrics is a wearable pulse oximeter. The device uses an optical sensor to detect the wearer’s oxygen saturation and pulse rate. A Bluetooth-connected companion app collects the data and lets patients track their metrics.
August 30, 2023

Source: Wellysis
Wellysis’s S-Patch Ex is a wearable ECG patch that monitors heart rhythms, intended for patients with recurrent symptoms such as shortness of breath, heart palpitations and chest pains. The device continuously captures ECG signals and sends data over Bluetooth to a companion mobile app.
September 8, 2023

Source: Hearing Health Matters
Hearing aids sold over the counter (OTC) and without a prescription can help democratize access to audiological care. However, setting up a pair of hearing aids without professional help can be difficult. The Intrisound Tuned Lumen 155 are self-fitting hearing aids with an AI-powered smartphone app that guides users through the setup process. The app also serves as a platform for users to troubleshoot problems and contact customer service.
Sep 14, 2023
The Transcutaneous Electrical Applicator (TEA) Device from Transtimulation Research is a non-implanted nerve stimulator that relieves abdominal pain for patients with Irritable Bowel Syndrome (IBS). The device stimulates the cranial and occipital nerves located on the back of a patient’s head. Patients can control the device via an interface on the device itself or with the Bluetooth-connected TEA IBS mobile app.
Sep 25, 2023

Source: Jinghao
Like the previously mentioned Tuned self-fitting hearing aids, these OTC hearing aids from Jinghao are adjusted by a patient via a Bluetooth-connected companion mobile app.
Oct 6, 2023
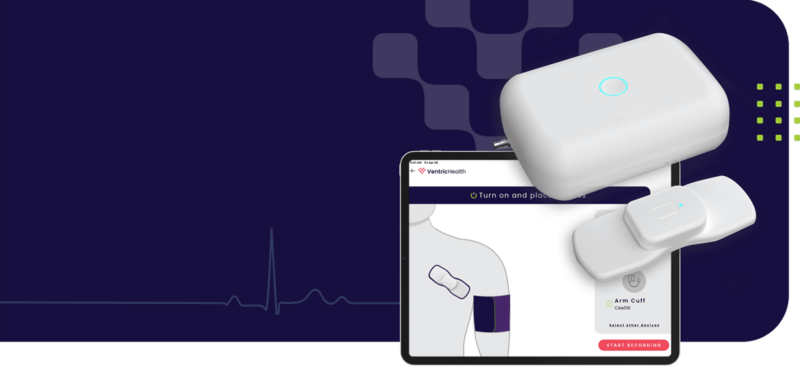
Source: Ventric Health
The Vivio System from Ventric Health is a medical device that can non-invasively diagnose heart failure. The device consists of an arm cuff, an ECG patch and an app that runs on a tablet, which guides the patient through setting up the system and connecting the two hardware components over Bluetooth. Once set up, the Vivio system can measure a patient’s left ventricular end-diastolic pressure in less than five minutes.
Nov 28, 2023

Source: Cue Health
Cue received De Novo clearance for a COVID-19 test that is integrated into their Cue Reader portable molecular testing platform. It is the first at-home COVID test granted marketing authorization by the FDA using traditional pre-market pathways, as opposed to prior COVID-19 at-home tests that came through Emergency Use Authorization (EUA).
The Cue COVID-19 test consists of a cartridge, which slots into the Cue Reader device, and a nasal swab used to take a sample. After the sample is collected, the swab is inserted through the cartridge and into the Cue Reader for analysis. Once the test is complete, results are sent over Bluetooth to the companion smartphone Cue Health App.

Source: Compumedics
Dec 8, 2023

Source: Nanowear
Wearable health technology company Nanowear received clearance for their SimpleSense Platform and SimpleSense-BP software application. Simplesense is a remote diagnostic platform that uses a cloth wrap with built-in sensors worn around the chest and over the shoulder to intermittently measure and collect data on a patient’s ECG, heart sounds, respiration rate and physical activity. The SimpleSense-BP app utilizes the data collected by the wrap to estimate a patient’s blood pressure.
Nanowear is a long-time Orthogonal client and friend. Read our case study on Nanowear.
_________________________________________________________________________
Looking for a partner to develop your Bluetooth enabled medical device? Orthogonal’s extensive library of edge cases, testing methods and mitigations will help you get the most out of Bluetooth communication for connected medical devices.
Related Posts

Article
Building Interoperability in SaMD: Tools and Best Practices
Article
Leveraging AI/ML in SaMD Development: Benefits and Challenges
Article
ISO 13485 Compliance Checklist for SaMD Development
Article
How to Conduct Post-Market Surveillance for SaMD