
Article
Roundtable Chapter 1 – The One Where the Device Was Only Half the Story
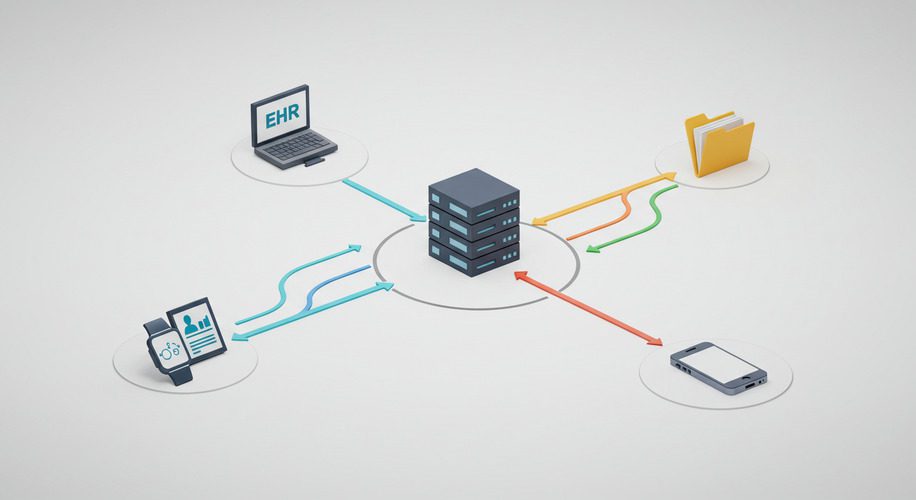
Real-world evidence (RWE) is transforming the way Software as a Medical Device (SaMD) solutions are validated, ensuring they are both effective and safe for use in diverse patient populations. Incorporating RWE into the validation process can strengthen regulatory submissions and improve post-market performance. This guide explores the benefits, sources, and best practices for using RWE in SaMD validation.
Real-world evidence refers to clinical data gathered outside of controlled clinical trials, such as from electronic health records (EHRs), wearable devices, and patient registries.
Related: How to Conduct Post-Market Surveillance for SaMD
Related: Leveraging AI/ML in SaMD Development: Benefits and Challenges
Related: ISO 13485 Compliance Checklist for SaMD Development
RWE provides actionable insights for refining algorithms and optimizing software performance.
By reflecting real-world conditions, RWE ensures SaMD solutions address actual patient needs effectively.
RWE-driven feedback loops enable continuous improvement and faster iteration cycles.
Incorporating real-world evidence (RWE) into SaMD validation is a powerful way to enhance safety, effectiveness, and regulatory success. By leveraging diverse data sources and aligning with best practices, you can ensure your SaMD delivers value in real-world healthcare settings.
For further insights, explore related articles:
Start using RWE to validate and refine your SaMD today, driving better outcomes and competitive advantage in the MedTech industry.
Related Posts

Article
Roundtable Chapter 1 – The One Where the Device Was Only Half the Story

Article
Roundtable Prologue – The One Where the Algorithm Couldn’t Sell Itself

Article
Managing Emerging Risks in SaMD: Strategies for 2025 and Beyond

Article
Emerging Opportunities in SaMD and MedTech for 2025