
Article
Harnessing IoT for Improved Compliance and Monitoring in SaMD
The World Health Organization (WHO) has established an online platform called Medical Devices Information System (MeDevIS), which reports that approximately 10,000 medical devices are used worldwide for the protection, prevention, diagnostics, treatment, and rehabilitation of health issues. Medical devices range from complex, expensive equipment like MRI machines to simple medical tools like stethoscopes to at-home diagnostic devices like pulse oximeters. According to recent market analysis from Fortune Business Insider, the global medical devices market was valued at $518.46 billion in 2023 and is projected to grow to $886.80 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 6.3% during this forecast period.
Over the last half-century, the amount of software used both in and around medical devices has dramatically increased. The last 20 years in particular have seen an acceleration of this trend, thanks to the emergence of the “Internet of Things” (IoT) and its corresponding parts: smartphones, wireless connectivity, cheaper and better sensors, cloud computing, big data and AI. These technologies are transforming how work gets done across almost every industry.
Software is a game-changer in healthcare and is disrupting almost every aspect of the sector, including therapeutics, genomics, drug discovery and development, bioinformatics, robotics, point-of-care diagnostics, virtual care, remote patient monitoring and even 3D printing.
One example of these technologies continuing the seismic shift in the administration and delivery of healthcare is how software has become an increasingly prevalent and crucial component of highly sophisticated medical devices.
Medical device software comes in four primary subclasses:
1. Software as a Medical Device (SaMD), which is standalone software that serves as a medical product in and of itself,
2. Software in a Medical Device (SiMD), or software that is part of a larger medical device, such as implanted software in medical equipment,
3. Software as an accessory to a medical device,
4. General purpose software that is not a medical device by itself.
Software as a Medical Device (SaMD) has seen particularly fast growth in recent years. Because software is far easier to change than hardware, it’s possible to evolve it faster through iterative development using fast feedback loops.
One way to look at SaMD’s growth is to monitor the rising use of Artificial Intelligence and Machine Learning (AI/ML) in medical devices. While not every SaMD uses AI/ML and not every device with AI/ML is a SaMD, there is a strong overlap between the two categories of medical devices. A 2020 article in Nature’s npj Digital Medicine identified 64 FDA-approved medical devices and algorithms that utilized AI/ML, and a 2021 article in The Lancet Digital Health identified 222 AI/ML medical devices approved in the USA and 240 devices in Europe. The FDA has been tracking the rapid growth of AI/ML-enabled medical devices in recent years. As of August 2024, the FDA had authorized 950 AI or machine learning-enabled medical devices.
Another way to look at the growth of SaMD is to see how many have been cleared by the FDA. As of October 2024, Orthogonal and SaMD industry consultants Brian Binkowski and Ritam Priya updated the list of FDA-cleared SaMD to 603 devices. We estimate that the range of total SaMD cleared by the FDA and on the U.S. market could be anywhere from 700 to nearly 5,000.
SaMD presents new opportunities and new challenges for both device companies and for regulators. It represents an area where new regulatory paradigms are being piloted and implemented to better enable medical device innovation while ensuring patient safety and clinical effectiveness.
In this introductory white paper, we provide an overview of Software as a Medical Device, explaining what it is, its benefits, which healthcare stakeholders stand to benefit the most from it (and why) and more.
According to the International Medical Device Regulators Forum (IMDRF), Software as a Medical Device is a class of software “intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device.[1]” This definition encompasses software and/or mobile apps intended to treat, diagnose, cure, mitigate, or prevent disease or other conditions.
SaMD’s defining feature is that it performs these medical functions without a need for actual hardware to be included in the FDA filing. All SaMD need computing power to run, but according to the FDA, computing sources such as the cloud, smartphones, databases and servers are not considered part of the device, as they are not proprietary systems. Rather, they are treated as Software of Unknown Provenance or as a produced service.
How SaMD interact with stand-alone physical devices varies. Some SaMD operate without a physical medical device; some are designed to work with a specific medical device (e.g., a SaMD companion app filed separately from the physical device); some SaMD are “device-agnostic” and are compatible with one or more medical devices that are filed separately (e.g., a diabetes management app usable with multiple manufacturers’ continuous glucose monitors).
Many people in the medical device industry use “SaMD” colloquially to refer to any software that is regulated as part of a connected medical device system. For the purposes of this white paper, our use of the term SaMD conforms to the IMDRF definition. As a firm, Orthogonal develops both SaMD that fall under the strict IMDRF, as well as connected medical device systems with software, which are often colloquially referred to as SaMD.
SaMD is not software that supports the functioning of medical device hardware. That is instead referred to as Software in a Medical Device (SiMD).
To determine if software qualifies as SaMD, answer yes to the following two questions:
1. Is my software a medical device? Is there a medical benefit to its use, and is there patient risk if it malfunctions or is used incorrectly?
2. Can my software work independently from another device? While SaMD can take data from another device, if your software supports the medical device, and that medical device does not work without your software, then your software is considered SiMD or part of a connected medical device system, not SaMD.
Some examples of the difference between SaMD and SiMD[2]:
• A mobile app that acts as a remote control for a headless device is not SaMD, because it serves as an accessory to the medical device.
• Software that helps radiologists and clinicians find and diagnose a cardiovascular condition by analyzing MRI scans is SaMD. The piece of software that turns on and controls the X-ray machine from the “inside” of the physical device is SiMD.
• A mobile application that takes input from a blood glucose meter and patient food log to provide insulin dosage recommendations for diabetes is SaMD if it communicates with multiple brands of glucose meters or accepts manual inputs. If it requires input from a specific glucose meter, then it is an accessory to that meter, and thus not SaMD. If the mobile app is the primary display for the glucometer, then it is part of the glucometer rather than an accessory, and thus not SaMD.
• A dedicated piece of software that monitors a mole for a given period of time to assess the risk of melanoma is SaMD.
• Software that analyzes a patient’s medical history and diagnostics data to determine the correct drug dosage is SaMD.
• Software which gathers, retrieves or organizes the actual medical data is not SaMD or SiMD. For example, an Electronic Health Record (EHR) system is neither SaMD nor SiMD. (We won’t discuss this last example in more depth because the FDA’s approach to the classification and regulation of EHR software could be the topic of its own white paper.)

The increasing adoption of SaMD can lead to improving patient outcomes faster. By automating aspects of care using the latest technologies and clinical evidence, SaMD can help accelerate the discovery, management, and treatment of a variety of medical conditions.
SaMD’s key characteristics include:
1. Improved health outcomes powered by data: SaMD can amplify the effectiveness of medical devices and existing treatment plans by enabling easy and fast collection of high-quality patient data, leading to better health outcomes. This characteristic is illustrated by the case studies to follow.
2. Faster feedback from data leading to faster innovation: SaMD enhances and builds on existing medical device functionality through software solutions that are faster and cheaper to update than hardware. With a connection to the wider Internet, including the Internet of Things (IoT), SaMD can tap into the latest technologies used to integrate and share health data across platforms, including the cloud, connected medical devices and smartphones, and use that data to guide innovation of the software product. On top of gathering data from online sources, SaMD can collect important feedback data from the user, including data generated by the patient using the SaMD, as well as patient-reported outcomes and experiences.
The flexibly of software plus the ability to gather real-time data and feedback allow developers to quickly iterate and improve SaMD over short Agile development cycles, known as “fast feedback loops.”
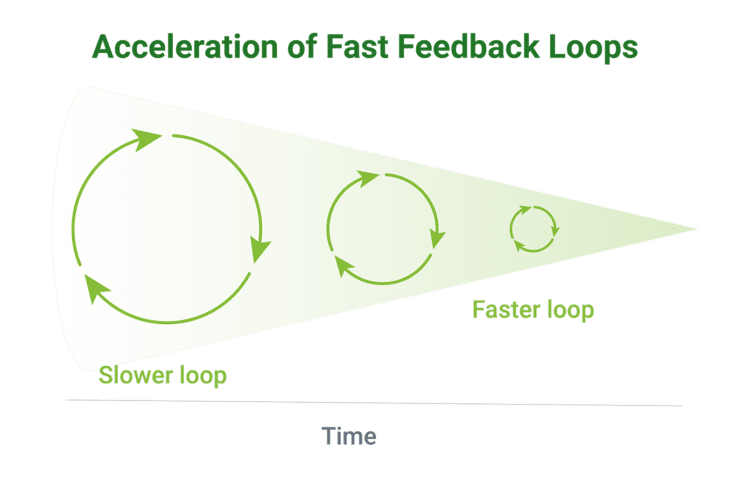

Before we dive deeper into what it can do, it’s important to remember that SaMD is a category of software, not a physical object. When we speak of SaMD, we refer to the group of medical device software products that meet SaMD functionality requirements.
SaMD “piggyback” on top of other physical connected technologies while leveraging their benefits. These technologies can include smaller, cheaper, and more sophisticated sensors; connectivity solutions such as 4G LTE and 5G cellular, Wi-Fi, and Bluetooth; increasingly powerful mobile devices such as smartphones and tablets which act as a “supercomputer” in the palm of one’s hand; and cloud services that enable distributed processing and data storage without the need for individual companies to build out their own physical storage spaces.
The more data SaMD can collect – from devices, sensors, smartphones, user feedback or activity and external conditions (including weather, pollution data and information based on patient location) – the more this data can be applied towards sophisticated algorithms that help deliver better care.
What’s more, SaMD not only collects critical data from patients, but also procures data from a wide variety of outside sources, combines it with other data, analyzes it, and then uses it automatically or presents it to users to act upon.
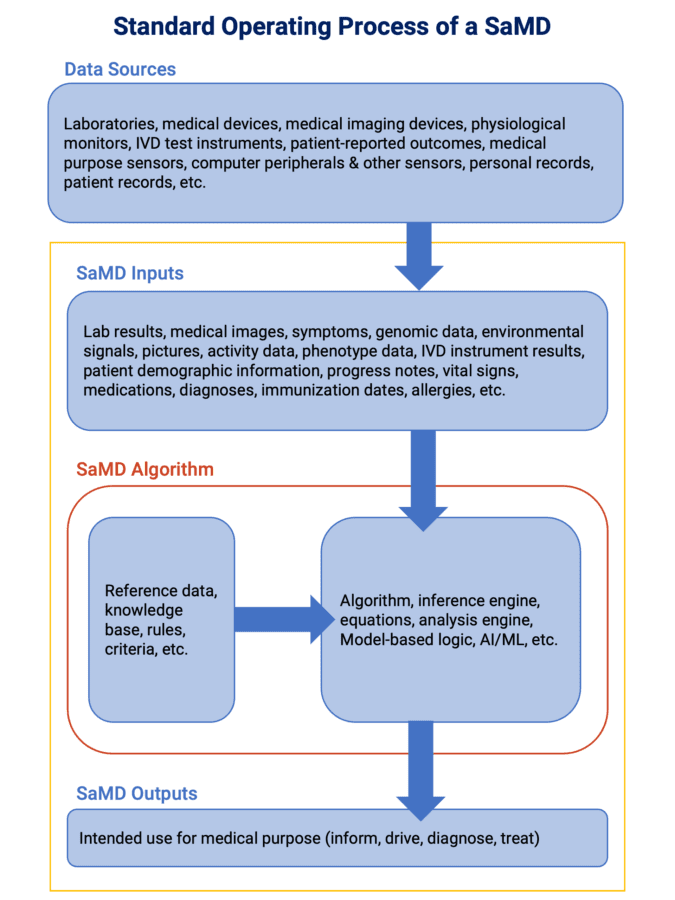
This data mobility enables SaMD to deliver more precise treatment options to patients and allows manufacturers to glean unique clinical insights, especially when combined with software technologies like AI and big data management tools.
State-of-the-art SaMD platforms can enhance the delivery and administration of care, reduce costs and improve health outcomes. This includes applications across three domains:
By leveraging complex algorithms, SaMD can accurately predict the likelihood of disease and aid in treatment decisions.
Patient benefits:
SaMD can help deliver personalized treatment plans, as well as reduce the time from diagnosis to treatment for patients with more complex diseases.
Lung cancer, for example, is difficult to detect, and most lung cancers are already at an advanced stage by the time they are discovered. SaMD can use smart algorithms capable of detecting anomalies that would otherwise go unnoticed in earlier CT scans, such as blood vessel problems or pleural disorders, increasing the survival rates among these patients.
Provider/clinician benefits:
By tapping into insights from SaMD, clinicians can gain a more holistic picture of a patient’s medical condition, history and real-time status, allowing them to make earlier, better and more precise diagnosis and treatment recommendations.
Example:
Ultromic’s EchoGo use AI to aid physicians in identifying coronary artery disease and other forms of heart failure from the examination of an electrocardiogram. EchoGo boasts a 90% accuracy in detecting heart failure with preserved ejection fraction.
SaMD systems can use data that comes from wearable sensors (e.g., a smartwatch, smart band or smart ring) to track multiple vital signs. SaMD monitors and uses this data to enable targeted recommendations and alerts for both patients and clinicians.
Patient benefits:
SaMD can help patients better monitor their condition by identifying triggers and providing real-time recommendations on actions ranging from drug dosages to physical activity to environmental conditions.
Provider/clinician benefits:
SaMD systems allow for remote monitoring of patients, such as ambulatory blood pressure monitoring. SaMD can collect and process data from sensors worn outside of the doctor’s office and detect subtle deviations that might flag important health issues. This data can help physicians get a better sense of patients’ blood pressure in their day-to-day lives and refine their treatment decisions.
Example:
PhysIQ is a proven, first-of-its-kind data analytics platform designed to process multiple vital signs from wearable sensors to create a personalized dynamic baseline. By mapping vital sign relationships, physIQ’s analytics detect subtle deviations that may be a precursor to disease exacerbation or change in health. PhysIQ is device-agnostic and can be used with any FDA-cleared chest-worn biosensors that record data in a compatible format.
SaMD that takes the form of Digital Therapeutics (DTx) plays a vital role in the treatment or mitigation of critical illnesses thanks to its ability to generate – and feed – highly-relevant clinical data into “other medical devices, medicinal products, general purpose actuators or other means of providing therapy to a human body.[3]”
Patient benefits:
DTx is often used as an aid in the treatment of chronic conditions or diseases. Take sleep apnea, for instance. According to the IMDRF, SaMD can “use the microphone of a smart device to detect interrupted breathing during sleep and sounds a tone to rouse the sleeper[4]” to prevent potentially serious effects.
Provider/clinician benefits:
DTx can enable clinicians to provide cutting-edge treatment to patients with complex, hard-to-treat diseases. For example, Akili’s EndeavorRx is a prescription video game for people with ADHD that uses specific sensory inputs to target and activate neural systems related to attention function.
Example:
metaMe Health is a prescription DTx company focused on the treatment of gastrointestinal conditions. The first DTx released on their platform was Regulora, an FDA-approved, all-digital, self-administered behavioral therapy for the treatment of patients with symptomatic irritable bowel syndrome (IBS).
Regulora is a digital hypnosis product with the potential to greatly scale affordable access to gut-directed hypnotherapy, a therapy that addresses the root causes of IBS. There are a limited number of clinicians, generally only found in select major urban medical centers, who are trained to deliver this treatment. In contrast, Regulora allows patients to self-administer a proven online hypnosis intervention from their own home on their own schedule.

The increasing importance of software, the increasing complexity of systems and the increasing speed of innovation are not unique to SaMD. They are part of a larger trend: more software, more connectivity, more interdependence and more data are shaping many areas of medical devices.
Over a decade ago, the Food & Drug Administration (FDA) recognized that their old paradigm of evaluating medical products wouldn’t suffice in a market of increasingly fast-paced innovation. Since then, they’ve been working to create a regulatory framework for medical devices and medical device software that prioritizes both patient safety and healthcare innovation.
Important steps taken by the FDA include:
• 2011’s Case for Quality program, where the FDA began working with healthcare stakeholders to “identify device manufacturers that consistently produced high-quality devices ” in order to promote device manufacturing best practices.
• 2017’s Software Precertification Program, which expanded on the Case for Quality program and applied it to medical device software, including SaMD, while emphasizing patient safety and innovation.
• The Digital Health Innovation Action Plan (2017), which aims to improve the federal agency’s digital health review process to provide timely access to safe and effective medical technologies.
• The alignment of U.S. medical device regulations from FDA 21 CFR Part 820 to ISO 13485:2016, a standard recognized by much of the rest of the world in the medical device market, and which emphasizes the FDA’s focus on risk management (2018).
• 2018’s reorganization of the FDA’s pre-market and post-market review process to address total product life cycle (TPLC) and “help facilitate information sharing to allow for more informed decisions, ensure process and policy consistency, and provide more straightforward and streamlined interactions.”
• 2022’s draft guidance on recommendations for Content of Human Factors Information in Medical Device Marketing Submissions, in recognition of the importance of human factors testing in balancing the benefits and risks of a connected medical device.
• The FDA’s recommendations for the instantiation of Predetermined Change Control Plans (PCCP) (2023) for medical devices that use AI/Machine Learning, potentially opening a new regulatory pathway for medical device software updates that does not require full resubmission.
• 2023’s guidance for pre submission of medical device software, information that developers can use to facilitate the FDA’s premarket review.
• Adoption of ISO 10993-1, “Biological evaluation of medical devices – Part 1: Evaluation and testing within a risk management process” (2023), guidance for preparing submissions of medical devices that come in contact with the body to determine if said contact results in adverse effects.
• Final guidance that supports a voluntary program for the Qualification of Medical Device Development Tools (2023), which are used in the evaluation of medical devices by the FDA’s Center of Devices and Radiological Health.
• 2023’s draft guidance on the FDA’s current best practices in selecting a predicate device for a 510(k) medical device submission.
• 2023’s final guidance on cybersecurity for medical devices, released in the same year that the FDA received additional statutory authority around cybersecurity from the Senate’s 2023 Omnibus Bill.
To help facilitate the tremendous potential of SaMD to increase both the quality and effectiveness of care, as well as the growth of general computing platforms and connectivity, the FDA has launched multiple initiatives that champion a concept widely used in many industries, broadly known as “Shift Left.”
According to Devopedia, “Shift Left” is the principle in development of “take[ing] a task that’s traditionally done at a later stage of the process and perform[ing] that task at earlier stages.[5]” By shifting actions such as testing from the end of development to earlier on in the process, it’s possible to identify “potential roadblocks and bottlenecks early on when there’s still scope to change and improve design.[6]” This has the obvious benefit of eliminating costs associated with fixing problems late in development and can lead to faster releases at a higher quality.
The established model of FDA submissions for SaMD – where review occurs after development – was borrowed directly from the submission process of physical devices and pharmaceuticals and does not have mechanisms to take advantage of the uniquely iterative nature of software products. To illustrate how the FDA has addressed this, we’ll examine its two foremost Shift Left initiatives: Digital Health Precertification (or Pre-Cert) and Predetermined Change Control Plans (PCCP).
Launched in 2017, the Pre-Cert pilot program represented a Shift Left from FDA clearance of a product at the end of development to FDA review of a company’s quality processes before development. Instead of evaluating a company’s single medical device or SaMD, the FDA instead reviewed companies holistically. Those companies that demonstrated their ability to develop safe and effective medical device software products were given a fast-track to market for individual devices with far less regulatory oversight.
Pre-Cert was warmly received by many in the medical device industry, who hoped it would lead to a change to the current model of FDA submissions. However, the U.S. Congress expressed concerns that the FDA had overstepped its congressionally authorized boundaries by pre-approving companies rather than approving individual devices. As a result, the FDA scaled back the Pre-Cert initiative and abandoned the concept of pre-approved companies. That said, Pre-Cert was a valuable learning experience for both industry and regulators and is likely informing future Shift Left initiatives from the FDA[7].
Similar to the Pre-Cert program, PCCP builds on the concept that demonstrating quality processes at the outset of and throughout the development process is just as if not more valuable than testing the final outcome. Currently, if developers want to make “significant” changes to previously cleared medical devices, they are required to resubmit the modified device to the FDA for a new review. A PCCP is an agreement between the FDA and a manufacturer of the specific, pre-defined changes that can be made to a medical device post-release without needing to refile. It places guardrails around the scope of what can be changed, as well as around the process of making those changes.
As an upfront agreement drafted at the beginning of a device’s lifecycle, the PCCP represents a Shift Left for manufacturers, as they no longer need to re-engage with the FDA for every change to a medical device. Orthogonal anticipates that PCCP will not only speed up the development process and save money for manufacturers, but will also allow regulators to refocus their time on other devices which would benefit from closer regulatory oversight.

As described earlier, SaMD’s defining characteristics are its ability to improve health outcomes through data and using fast feedback loops powered by that data to drive faster product iteration and ultimately faster innovation.
While this will undoubtedly move the industry forward, it underlies the fundamental challenge many builders of SaMD face: How do you integrate modern product management and software engineering tools and techniques designed for a fast-changing, ever-evolving digital world with a structured and rigorous focus on patient safety, clinical effectiveness and regulatory compliance?
It’s our opinion that companies that can navigate this integration will successfully reap the rewards; those that cannot will be left behind.
The following techniques lead to faster, cheaper feedback loops while ensuring patient safety and product innovation:
• Agile development methodologies like verification, test automation, real-world data collection, Lean UX and Human Factors, risk management and document automation integrated as part of a total product lifecycle (TPLC) approach.
• Risk-based software segregation to determine where developers can iterate freely and where they need Institutional Review Board (IRB) oversight.
• Continuous IRB reviews for faster feedback in areas of high risk to patients.
• Faster, more frequent formative Human Factors testing, both during development and in-market, coupled with real-world performance analytics to understand actual user interaction with devices in production.
• Faster, cheaper and more effective clinical validation.
The fundamental challenge faced by regulators is how to ensure patient safety and clinical effectiveness while enabling faster innovation. Fortunately for our industry, the FDA is interested in working with us to enable these kinds of optimizations, and actively encourages MedTech to bring new ideas on how to “build a better mousetrap.” The Pre-cert Program, the Case for Quality, the PCCP pathway and an increased focus on TPLC are all great illustrations of this support.
It’s also important to remember that there is no “one size fits all” approach to developing SaMD. Some approaches may be better suited to nimble startups, while others are better suited to the global MedTech “giants.”
For larger companies, starting with pilot programs to implement best practices as well as fast feedback loops throughout the product life cycle may be the best approach. In some cases, this may involve making changes to a company’s quality management system (QMS). These changes are generally easier for firms with existing QMS systems that allow for “loose coupling,” leaving more room for methodology decisions to be made at the product level than at an enterprise level.
“Software is eating the world,” according to Mark Andreesen in his highly influential 2011 op-ed in the Wall Street Journal. In the years since his prediction, we’ve seen a broad economic shift towards technology and software across every sector. All kinds of companies are investing in SaMD, including non-device medical software companies, pharmaceutical companies, companies with non-connected medical device software and consumer electronic firms investing in health platforms.
If you’re a company operating in a software space that may cross into FDA-regulated areas but are intentionally staying below the FDA’s regulatory radar, we highly urge you to reconsider. While there are advantages to a “non-medical device” strategy, it is possible that some of your competitors will decide to take the leap and pursue getting FDA clearance for medical device software, opening new avenues of revenue and strategic possibilities. Your competitors may be able to make medical claims that you cannot and use this to their strategic advantage to sell in healthcare. They may also get insurance reimbursement much more easily and at higher rates. All of this could raise their profitability and valuation.
Entering the FDA-regulated world is a large step and does require investment of time, money and resources. But it is not an all-or-nothing proposition. There are ways to limit the initial investment for companies that want to try the regulated software approach before scaling it. With careful planning, it’s possible to move into the device software space without converting all existing clinical products into regulated medical devices, preserving the speed at which non-device products are developed.
If you develop SiMD but not SaMD, all the same pressures and dynamics of the IoT apply: more connectivity, more software, more change, more complex systems.
As evidenced by the FDA’s evolution in regulating SaMD medical devices (the transition to ISO 13485:2016, OPEQ, Case for Quality, Pre-Cert and PCCP), their expectation is these new regulatory paradigms will move throughout the system.
Companies that traditionally operate without software (pharmaceutical companies, or device makers of software-less hip or shoulder implants, etc.), are also impacted by the increase in value that software creates. These devices become “smart” as soon as a sensor is put into them (for examples of this, see the FDA’s 2018 workshop on Orthopaedic Sensing, Measuring, and Advanced Reporting Technology (SMART) Devices).
If you are a pharmaceutical company, the same pressures to demonstrate real-world performance, improve adherence and to create precision therapies will eventually affect you, just as they are currently affecting medical device companies.
We believe that pharma companies will eventually be judged and compensated based on real-world performance of their therapies, rather than on purely clinical trial outcomes. At the same time, Digital Therapeutics have the potential to demonstrate outcomes in clinical trials that are as good as or better than drugs treating the same conditions.
Some DTx can also work in combination with drugs to provide even better outcomes than the individual drug or DTx alone. Because they are designed to collect data while interacting with the patient, DTx could make it easier and more cost effective to monitor performance and influence adherence. It might also more closely link pharma companies to the recipients of their drugs.
Digital integration may lead to greater adoption and compliance among patients, which could influence better patient outcomes as well as more profitable reimbursement for the pharma firm. For these reasons, the digital transformation of pharmaceuticals may not just be inevitable, but could also be beneficial for patients, providers and pharma itself.
Since the original publication of this white paper, Software as a Medical Device has evolved from a niche category of medical devices to a mainstay in the treatment of many conditions. With hundreds or perhaps even thousands of devices already cleared by the FDA and more coming every year, SaMD is a trend that every medical device manufacture needs to have on their radar.
Companies that have chosen to ignore this new reality face a choice. They can continue to ignore the rise of SaMD by sticking their heads in the sand and risk losing market share to competitors. Or they can rethink their position on software’s value-add to medical devices and start taking a close look at SaMD to determine if there are ways it could improve their products, profitability and patients’ lives.
SaMD has hit its stride. It has the support of industry, providers, patients and regulators. It is time to start taking SaMD seriously as both an opportunity and a challenge to traditional medical device manufactures.
SaMD is a key part of the future of healthcare. How you act upon that future is up to you.
1.https://www.imdrf.org/sites/default/files/docs/imdrf/final/technical/imdrf-tech-131209-samd-key-definitions-140901.pdf
2.For simplicity reasons, we’ve glossed over the extreme edge case of SaMD in SiMD (e.g., a SaMD algorithm sitting inside firmware of a separate, physical medical device).
3.https://www.imdrf.org/sites/default/files/docs/imdrf/final/technical/imdrf-tech-140918-samd-framework-risk-categorization-141013.pdf
4.Ibid
5.https://devopedia.org/shift-left
6.Ibid
Orthogonal is a software development consulting firm that improves patient outcomes faster by helping MedTech firms accelerate their development of Software as a Medical Device (SaMD), Digital Therapeutics (DTx) and connected medical device systems. We do this by fusing modern software development and product management tools and techniques developed in other industries (such as Agile, Lean Startup, DevOps, User-Centered Design and Systems Thinking) with the regulated focus on safety and effectiveness that is at the heart of the medical device sector.
For almost fifteen years, we’ve helped a wide variety of firms in MedTech, Pharma and consumer electronics develop and bring their regulated/connected devices to market including Tandem Diabetes Care, Quidel, Medtronic, Novo Nordisk, Eli Lilly, physIQ, Nanowear, Google and Bose.
Website: https://orthogonal.io/
LinkedIn: https://www.linkedin.com/company/orthogonal
Twitter: @orthogonal_io
Our favorite inbound messages after we publish a white paper do not start with, “Great white paper. Keep up the good work.” Our favorites are the ones that start with, “I read your white paper, and I disagree when you say X because you are not taking Y and Z into consideration.”
Our ideas are ultimately strengthened by open, candid conversations. Do you agree with our take? Disagree? Have questions? Have you read an article that we should read? Feel free to reach out to Orthogonal’s CEO, Bernhard Kappe and Randy Horton, Chief Solutions Officer.
In return, we will look for ways to share more white papers, personalized insights, content and introductions that are highly relevant to your work.
Bernhard Kappe, Chief Executive Officer and Founder
For over a decade, Bernhard has provided thought leadership and innovation in the fields of Software as a Medical Device (SaMD), Digital Therapeutics (DTx) and connected medical device systems. As a leader in the MedTech industry, Bernhard has a passion for launching successful medical device software that makes a difference for providers and patients, as well as helping companies deliver more from their innovation pipelines. He’s the author of the eBook Agile in an FDA Regulated Environment and a co-author of the AAMI Consensus Report on cloud computing for medical devices. Bernhard was the founder of the Chicago Product Management Association (ChiPMA) and the Chicago Lean Startup Challenge. He earned a Bachelor’s and Master’s in mathematics from the University of Pennsylvania, and a Bachelor’s of Science and Economics from the Wharton School of Business.
Email: [email protected]
LinkedIn: https://www.linkedin.com/in/bernhardkappe/
Phone: (312) 372-1058
Brett Stewart, Principal Solutions Architect
Brett is a technology architect responsible for solutions engineering for a wide range of SaMD client projects and system architectures. He brings over 20 years of experience delivering enterprise and mobile software solutions in industries ranging from diabetes care to national intelligence, where he developed his knowledge on how to engineer systems to capture core competencies and allow for flexibility. Brett worked as the architectural and operational lead for a medication adherence app that reached the #1 ranking in the Health and Wellness category on the Apple App Store.
Email: [email protected]
LinkedIn: https://www.linkedin.com/in/voidstar/
Randy Horton, Chief Solutions Officer
Randy Horton is Chief Solutions Officer at Orthogonal, a software consulting firm that improves patient outcomes faster by helping MedTech firms accelerate their development pipelines for Software as a Medical Device (SaMD), digital therapeutics (DTx) and connected medical device systems. Orthogonal makes that acceleration happen by fusing modern software engineering and product management tools and techniques (e.g., Agile, Lean Startup, User-Centered Design and Systems Thinking) with the regulated focus on device safety and effectiveness that is at the heart of MedTech.
Horton serves as Co-Chair for AAMI’s Cloud Computing Working Group, as well as AAMI CR:510(2021) and the in-process Technical Information Report #115, all of which address how to safely move medical device computing functions into the cloud. He is a frequent speaker at conferences and webinars, including events hosted by AdvaMed, AAMI, HLTH, RAPS and the Human Factors and Ergonomics Society (HFES).
Email: [email protected]
LinkedIn: https://www.linkedin.com/in/randyhorton/
Phone: (312) 433-9437
Related Posts

Article
Harnessing IoT for Improved Compliance and Monitoring in SaMD
Article
Regulatory Innovation in SaMD: How Global Standards Are Evolving

Article
Implementing Real-Time Data Analytics in SaMD Solutions

Article
Navigating FDA’s Proposed AI/ML Framework for SaMD