Launching Software as a Medical Device (SaMD) is a complex process that requires careful planning, regulatory adherence, and innovative execution. Examining successful SaMD launches offers valuable insights into best practices and strategies that you can apply to your own projects. This case study highlights two exemplary SaMD launches and the lessons learned from their success.
Case Study 1: AI-Powered Imaging Tool for Diagnostics
Overview
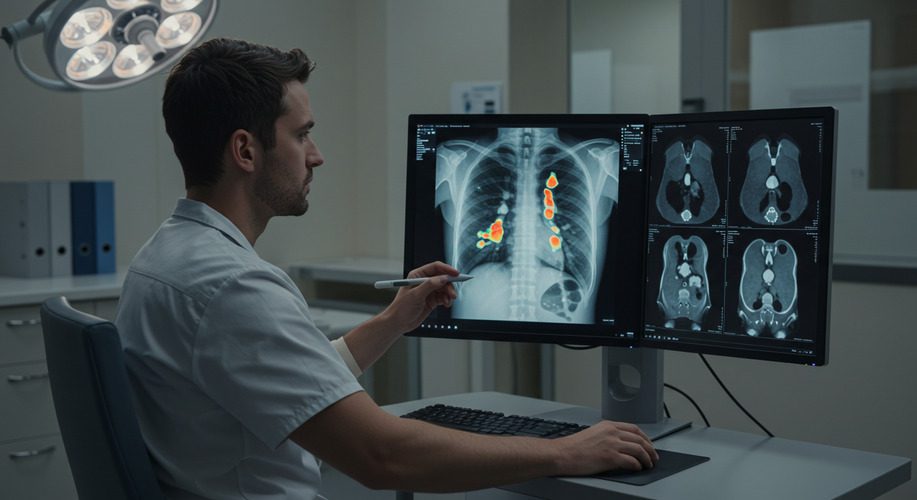
A MedTech startup developed an AI-powered imaging tool designed to detect early-stage lung cancer in radiology scans. The solution integrated seamlessly with existing hospital systems and provided real-time diagnostic support to radiologists.
Key Strategies
Early Regulatory Engagement:
- The company engaged with the FDA early in development, using the pre-submission process to clarify regulatory expectations.
- Adherence to ISO 13485 and IEC 62304 ensured a strong foundation for compliance.
Leveraging AI for Accuracy:
- Robust training datasets with diverse imaging samples reduced bias and improved diagnostic accuracy.
- AI explainability tools helped build trust with users and regulators.
Seamless Interoperability:
- Used HL7/FHIR standards to ensure compatibility with major electronic health record (EHR) systems.
- Reduced onboarding friction for healthcare providers.
Lessons Learned:
- Engage regulators early to streamline the approval process.
- Prioritize interoperability to maximize adoption.
- Invest in AI transparency to gain user trust.
Related: Building Interoperability in SaMD: Tools and Best Practices
Case Study 2: Remote Monitoring SaMD with IoT Integration
Overview
A global MedTech company launched a remote monitoring platform for chronic heart failure patients. The SaMD used IoT-enabled wearable devices to track patient vitals and provide real-time alerts to clinicians.
Key Strategies
Real-World Evidence (RWE) Integration:
- Leveraged RWE during validation to demonstrate effectiveness across diverse patient populations.
- Collaborated with healthcare providers to gather post-market data for iterative improvements.
Cloud-First Architecture:
- Built a secure, scalable cloud platform to support real-time data transmission and analytics.
- Ensured compliance with GDPR and HIPAA for data security.
Patient-Centric Design:
- Focused on usability by conducting extensive patient testing.
- Integrated mobile alerts and simple dashboards to increase engagement and adherence.
Lessons Learned:
- Use RWE to validate effectiveness and build credibility.
- Prioritize patient-centric design for better engagement.
- Ensure scalability and security with cloud-based solutions.
Related: How to Use Real-World Evidence (RWE) in SaMD Validation
Best Practices for a Successful SaMD Launch
1. Develop a Comprehensive Launch Plan
- Define clear objectives, milestones, and success metrics.
- Include regulatory milestones and marketing strategies.
2. Focus on Early Stakeholder Engagement
- Collaborate with regulators, clinicians, and end-users during development.
- Address their feedback to ensure your SaMD meets real-world needs.
3. Prioritize Interoperability
- Ensure compatibility with EHRs and IoT devices to maximize usability.
- Adopt standards like HL7 and FHIR for seamless integration.
4. Leverage Post-Market Surveillance
- Use PMS tools to gather insights and continuously improve your SaMD.
- Monitor performance and user feedback to identify potential enhancements.
Related: How to Conduct Post-Market Surveillance for SaMD (Advanced Guide)
Conclusion
The success of a SaMD launch hinges on strategic planning, stakeholder collaboration, and a focus on user-centric design. By examining these case studies and implementing the lessons learned, you can streamline your own SaMD launch process and achieve impactful results.
For further insights, explore related articles:
Apply these strategies to ensure your SaMD launch not only meets regulatory requirements but also exceeds user expectations and drives meaningful outcomes in healthcare innovation.