
Article
Roundup: Bluetooth Medical Devices Cleared by FDA in 2024
Exactly how many Software as a Medical Devices (SaMD) have been officially cleared by the FDA and reached the market? The answer to that question that I’ve given and heard from others over the last four years is, “Gee, I dunno. I’ve never seen that list compiled and I’m not sure you can get it right out of the FDA’s public databases.”
In the absence of an official list, I, Randy Horton, Chief Solutions Officer here at Orthogonal, Brian Binkowski and Ritam Priya, SaMD consultants with several decades of collective of industry experience, have started our own list – and we’d like your help in building and further refining it. View our current draft list in full here: The Ultimate Running List of FDA-Cleared Software as a Medical Device (SaMD).
The International Medical Device Regulatory Forum (IMDRF) defines Software as a Medical Device as “software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device.” This IMDRF definition, recognized by the FDA, includes the following clarifying details:
SaMD has grown to be one of the hottest topics in medical devices over the last decade, with Google Trends reporting that search interest in the U.S. reached an all-time high in 2023.
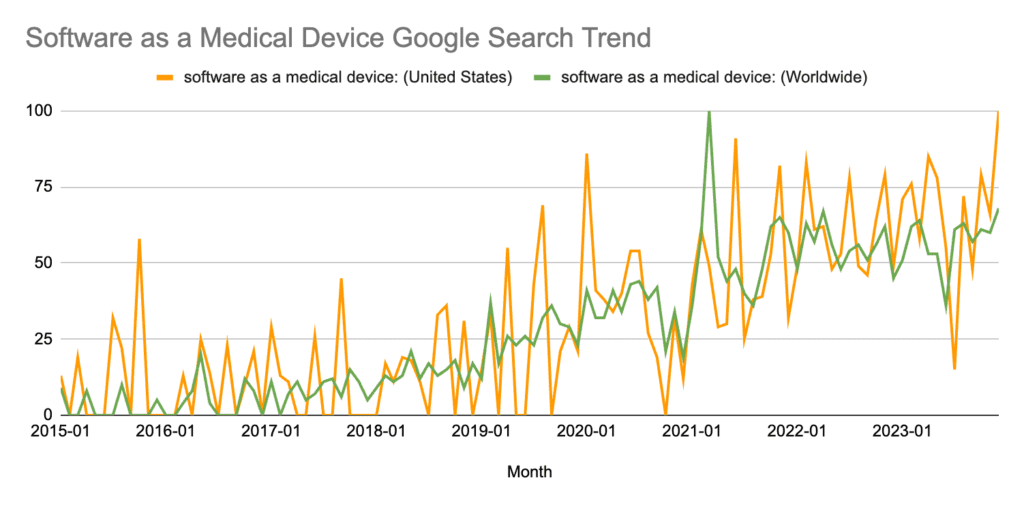
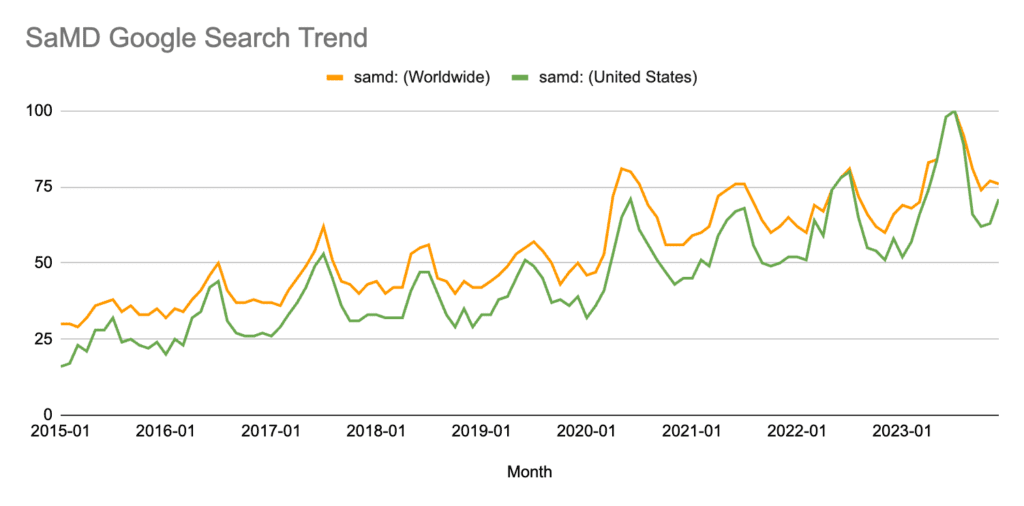
*Numbers represent search interest relative to the highest point on the chart for the given region and time. A value of 100 is the peak popularity for the term. A value of 50 means that the term is half as popular. A score of 0 means there was not enough data for this term. This article contains more information on interpreting Google Trends data.
Despite the rising awareness of and interest in SaMD among stakeholders in the medical device industry, and the many options currently available to patients and providers, we know of no list that catalogs all FDA-cleared SaMD out on the market to date.
The closest we have seen so far is the FDA Center for Devices and Radiological Health’s effort to document the number of devices they have cleared that utilize AI/Machine Learning (ML). This list was a useful starting point for our own efforts, but not every item on CDRH’s AI/ML list fits the criteria of SaMD. Though many SaMD utilize AI/ML, by definition SaMD do not have to include such algorithms. Likewise, there are many medical devices on the market which use AI/ML but do not qualify as SaMD due to the AI/ML algorithms being part of a larger system that includes physical or hardware-based components.
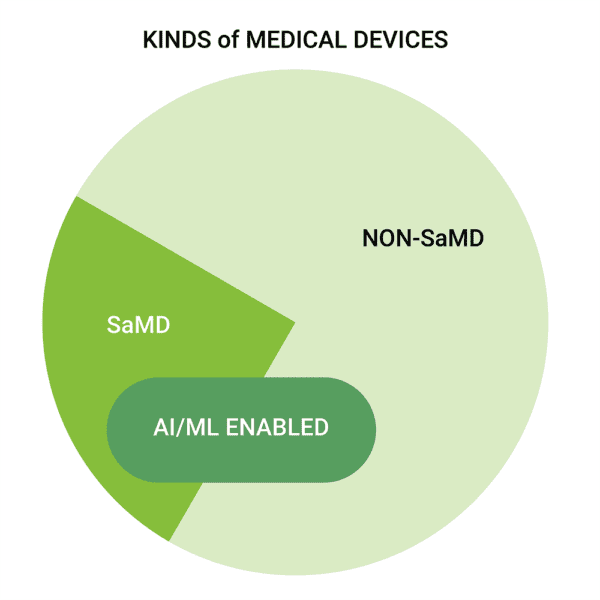
Not all medical devices using AI/ML are SaMD, and not all SaMD use AI/ML
Brian, Ritam and I anticipated that crafting our own FDA-cleared SaMD list from scratch would be a major endeavor. We were pleasantly surprised to discover that our task was far less challenging than we had imagined.
Brian, Ritam and I searched publicly available FDA databases to create the current version of our list of FDA-cleared SaMD on the market, found here: List of 483 FDA-Cleared Software as a Medical Device (SaMD). First, we pruned down the aforementioned AI/ML device list to only include devices that met our criteria of SaMD as defined by the IMDRF. We then added in data from exports of device data from 2022 and 2023 data, sourced from the FDA’s archive page for premarket submissions. Finally, we sourced pre-2022 FDA data by using queries and exports from Basil Systems, a commercial Software-as-a-Service vendor offering enhanced access to the FDA’s publicly available medical device datasets through a proprietary interface and a suite of value-added enhancements and harmonizations to the FDA’s published device data.
Our methodology for identifying SaMD in these datasets was to look for all devices whose “PHYSICAL STATES” [sic] related to stand-alone software, software device or software application. Devices with identified “PHYSICAL STATES” of stand-alone software were then combined to produce a list of associated product codes. We then used those product codes to look across other databases of AI/ML products as well as Basil Systems’s regulatory search to find stand-alone software and SaMD that we may have missed.
The list that came out of this process currently includes 483 devices. However, we believe there are many more SaMD out on the market that were not classified in the databases we searched under the criteria we described above and need to be added to our list. Furthermore, the data in the FDA databases we used could possibly include false positives (i.e., devices that are incorrectly classified and do, in fact, include a physical component.) Thus, we’d like your help to further build and refine our list!
Please take a look at our current list. If you notice a product is missing, or incorrectly listed, let us know! You can submit findings via this form, or by emailing us at [email protected].
With your help, we’ll have a list that fills a noticeable information gap with accurate data, valuable to anyone looking to improve patient outcomes faster by accelerating the development of SaMD.
This list is crowdsourced. Thank you to our crowd!
The initial development and ongoing buildout of this list rely heavily on those who read this blog, reviewed the Ultimate Running List of FDA-Cleared Software as a Medical Device (SaMD) and pointed out SaMD clearances missing from our list. We want to thank them here and ask everyone to take inspiration from them to help build this list!
Special thanks go to Greg Stern, Marc Neubauer, Karl Hess, Susumu Nozawa, Lindsay Ayearst, Hans De Leenheer, Adam Heroux, Mike Attili, Mike Nelson, Jacque Engle, Tina Lochner.
Randy Horton, Chief Solutions Officer, Orthogonal
Randy Horton is Chief Solutions Officer at Orthogonal, a software consulting firm that improves patient outcomes faster by helping MedTech firms accelerate their development pipelines for Software as a Medical Device (SaMD), digital therapeutics (DTx) and connected medical device systems. Orthogonal makes that acceleration happen by fusing modern software engineering and product management tools and techniques (e.g., Agile, Lean Startup, User-Centered Design and Systems Thinking) with the regulated focus on device safety and effectiveness that is at the heart of MedTech.
Horton serves as Co-Chair for AAMI’s Cloud Computing Working Group, as well as AAMI CR:510(2021) and the in-process Technical Information Report #115, all of which address how to safely move medical device computing functions into the cloud. He is a frequent speaker at conferences and webinars, including events hosted by AdvaMed, AAMI, HLTH, RAPS and the Human Factors and Ergonomics Society (HFES).
Brian Binkowski has over 10 years of medical device quality experience working for companies such as Boston Scientific, Medtronic, and DTx startup Twill. Brian is an accomplished and impressive “friend of Orthogonal.” He shares our belief that the MedTech industry can rapidly improve patient outcomes by adopting fast feedback loop techniques to the development and enhancement of Software as a Medical Device (SaMD), DTx and connected medical device systems.
Ritam Priya is a seasoned MedTech leader who has over 15+ years of experience in the medical device and digital health industry. She has worked for companies like Baxter Healthcare and Johnson & Johnson. Ritam enjoys working on innovative medical products; some of her most recent experience has been with digital health startup companies like Eko Devices, Beddr, Nocimed (now Aclarion) and Aktiia, where she has successfully led the quality and regulatory team. In her free time, Ritam enjoys reading, pottery, hiking, traveling and gardening.
Related Posts

Article
Roundup: Bluetooth Medical Devices Cleared by FDA in 2024

Article
SaMD Cleared by the FDA: The Ultimate Running List
Article
Roundup: Bluetooth Medical Devices Cleared by FDA in 2023

White Paper
Software as a Medical Device (SaMD): What It Is & Why It Matters